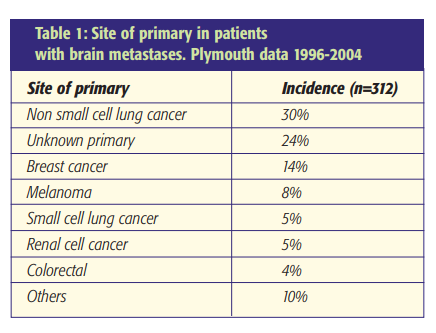
Incidence
The incidence of cerebral metastases is variably reported with 14.3 per 100,000 per annum in a Scottish population study [1]. Overall, a third of patients with cancer develop intracranial metastases. The commonest primary tumours arise from the lung and breast. Melanomas, renal cell carcinomas and gastrointestinal tract malignancies account for most of the remainder (Table 1). MRI and post-mortem studies indicate that at least 50% of patients have multiple lesions. Around 80% of intracranial metastases are supratentorial and 20% occur in the posterior fossa [2].
About 20% of intracranial metastases are precocious or synchronous presenting before or within two months of the primary tumour. Early presentation with intracranial metastases appears to be associated with poorer outcomes and frequently occurs in association with small cell carcinomas of the lung [3]. The remaining 80% of patients present with metachronous tumours at least two months after the primary. This is commonly observed in patients with breast cancer and may occur several years after the primary presentation [2].
Presentation
Patients with intracranial metastases have a spectrum of clinical presentations that can be classified as shown below [2].
- Raised intracranial pressure – due to tumour, oedema or hydrocephalus (c25%)
- Focal neurological signs – these are dependent upon tumour location and include cognitive deficits (c50%)
- Focal or generalised seizures (c15%)
- Asymptomatic – detected on radiological screening investigations (c10%)
Imaging
Contrast enhanced T1 weighted MRI scanning is the clinical investigation of choice. Double or even triple contrast dosing has been recommended to increase the sensitivity of the investigation. Metastases usually appear as well-circumscribed enhancing intra-axial lesions with peri-tumoural oedema. Supratentorial metastases are frequently located at the grey-white matter interface in the distribution of the middle cerebral artery. They may appear as a homogeneous or heterogenous mass with areas of cyst formation. Occasionally intraventricular lesions are evident. The differential diagnosis includes a primary brain tumour and sometimes brain abscesses. Dural-based metastases can occur with a differential diagnosis including meningioma. SPECT and PET studies provide physiological evidence of a hypermetabolic focus characteristic of malignancy. This may be helpful in distinguishing neoplasia from a cerebral abscess or radiation necrosis. MR spectroscopy and functional imaging techniques are not yet widely available but offer promise in establishing a non-invasive diagnosis and facilitating treatment approaches.
Management
Patients presenting with suspected intracranial metastases require a diagnostic work-up. MRI scanning is used to assess the number and location of intracranial metastases. If the patient has a known primary tumour, CT imaging is performed to assess the extent of local and extracranial metastatic disease. If the intracranial disease is precocious a full clinical examination and a CT thorax, abdomen and pelvis is performed to try to identify a primary lesion. A lung primary is often visible on chest radiograph. In the absence of a diagnosis, a biopsy of any extracranial disease is usually performed in preference to an intracranial procedure. Given a diagnosis of intracranial metastatic disease a variety of therapeutic options are available. The use of steroids is widely practised whilst the relative roles of surgery, whole brain radiotherapy and stereotactic radiosurgery are complex. The evidence for these treatments is reviewed below.
Steroids and Whole Brain Radiotherapy (WBRT)
If no treatment is initiated the median survival of patients with brain metastases is approximately one month from the time of diagnosis. Non-randomised studies suggest that this can be increased to two months if steroids are used and three to six months if WBRT is also given [4,5,6]. Steroids and WBRT have therefore been the mainstay palliative treatments for patients with cerebral metastases for more than 50 years [7]. WBRT is usually delivered either as 20 Gy in five fractions over one week or 30 Gy in 10 fractions over two weeks. However, in the small group of patients with a relatively good prognosis (those in Class 1 in Figure 1), it may be appropriate to give a more prolonged fractionation schedule to reduce the risk of late neurocognitive dysfunction.
Surgical excision of a solitary intracranial metastasis
Hart et al have reviewed the addition of surgical resection to the armamentarium in treating patients with solitary intracranial metastases [8]. Although there are a number of controlled trials, generally the number of patients recruited is small, and so significant differences in survival may be missed. Thus general applicability of these requires an informed, thoughtful approach.
Patchell et al randomised 48 patients with solitary metastases in a single centre [3]. Twenty-five underwent surgery + WBRT and 23 had WBRT alone. The survival hazards ratio (HR) 0.50 [95% CI:0.26, 0.95] favoured the surgical group with median survivals of 40 weeks in the surgery + WBRT group and 15 weeks in the WBRT alone group. In addition, functional independence was much better preserved in the surgical group (38 weeks versus 8 weeks P<0.005). Local or distant intracranial recurrence occurred in 20% of the surgical group and 52% of the WBRT alone group (P<0.02). Overall 71% of the surgery + WBRT cases who died (15/21) and 50% (11/22) of the WBRT alone cases died from systemic causes. It should be noted that the primary tumour was a non-small cell carcinoma of the lung in 37/48 (77%) cases and the high prevalence of this radioresistant tumour may bias the results in favour of surgery.
A Dutch trial (n=63) also showed a trend toward improvement in outcome in the surgery + WBRT group (10 months median survival) compared with WBRT alone (6 months median survival) although this did not reach statistical significance; survival HR 0.57 [95% CI:0.30,1.06] [9].
The Canadian multicentre trial recruited 84 out of an eligible 145 cases to a trial comparing surgery + WBRT with WBRT alone. In contrast to the other RCTs, a non-significant trend toward improved outcome with WBRT alone (n=43) rather than surgery + WBRT (n=41) was reported; survival HR 1.39 [95% CI:0.76, 2.55] [10]. These different findings compared with the reports of Patchell et al and Vecht et al may be due to different inclusion/exclusion criteria. Twenty-one percent of patients had a KPS of 50-60 and would have been excluded by Patchell et al and probably by Vecht et al. A higher proportion of patients had extracranial metastatic disease at the time of randomisation compared with the Patchell et al and Vecht et al studies (45% vs. 37.5% and 31.7% respectively). Fewer patients had non-small cell lung carcinoma (53.6%) compared with 77% in the Patchell study. The inclusion of such patients may have conferred a bias favouring WBRT. In addition the exclusion of 59 eligible cases questions the widespread applicability of the findings.
In summary, the evidence base appears to support the role of surgery in treating patients with accessible solitary metastases who have a relatively good performance status (KPS>70) with no or controlled extracranial disease.
Over the past decade frameless stereotactic neuronavigation has developed and provides the surgeon with the confidence to perform an accurately located minicraniotomy (3cm diameter) directed at tumour excision. A microscope assisted trans-sulcal or trans-gyral approach can then be performed to locate the tumour. A clear plane of dissection is usually achieved permitting removal of the lesion. For larger metastases, the cavitating ultrasonic aspirator can help debulk the lesion in parallel with progressive circumferential dissection at the tumour/ brain interface. Stereotactic biopsy is only performed where the tumour is considered inaccessible or the clinical status does not warrant craniotomy.
WBRT after surgical resection of a solitary metastasis
Evidence to support the use of adjuvant WBRT after resection of a solitary metastasis was reported in a further study by Patchell et al [11]. Forty-six patients were randomised to surgery and 49 to surgery + WBRT. The dose of WBRT was high, at 50 [4]. Gy in 28 fractions, in an attempt to reduce the risk of neurological sequelae. Recurrence of intracranial disease occurred in 70% of those not receiving postoperative WBRT versus 18% in the surgery + WBRT group (P<0.001) indicating that the addition of WBRT seemed to afford efficacious intracranial tumour control. On an intention to treat analysis WBRT did not confer a significant survival benefit (43 vs. 48 weeks; P=0.39; RR of death 0.91, 95% CI 0.59-1.40). However, the survival data are confounded by the fact that some patients crossed over and received WBRT on disease recurrence. Additionally, in the surgery alone group, 44% of patients were deemed to have died as a result of neurological disease compared with 14% in the combined treatment arm. Although it may be considered that this study provides Class I evidence to support the use of WBRT as an adjunct to surgical resection, others have argued that WBRT may not be necessary in all patients as this did not significantly improve overall survival, and duration of functional independence [12].
WBRT + SRS boost
1. Patients with unresectable intracranial metastases
Most patients with intracranial metastases are not suitable candidates for surgical resection due to the multiplicity of lesions, active extracranial disease and poor performance status. The majority of such patients with good performance status receive WBRT. Andrews et al reported a large RCT (n=331) addressing whether a SRS boost (Gamma knife or LINAC) improved outcome in patients with 1 to 3 intracranial metastases and a KPS≥70.13 For patients with solitary lesions, WBRT + SRS boost (n=92) increased survival from 4.9 months to 6.5 months (P=0.0393) compared with WBRT alone (n=94). A difference in survival was not identified for patients with multiple intracranial metastases. However, WBRT + SRS boost significantly enhanced or stabilised KPS status, enabled reduced steroid use and improved local control at follow-up across both the solitary and multiple metastases groups. The addition of an SRS boost to WBRT is therefore worthy of consideration in patients with up to 3 metastases and functional independence.
2. Patients who have undergone surgical resection
The rationale to treat patients who have undergone resection of a solitary metastasis with WBRT + SRS boost seems a logical application of the evidence from patients with unresectable metastases [13]. However, class I evidence to compare the outcome between patients who have received surgery + WBRT and surgery + WBRT SRS boost does not exist.
Is SRS an effective alternative to surgical resection?
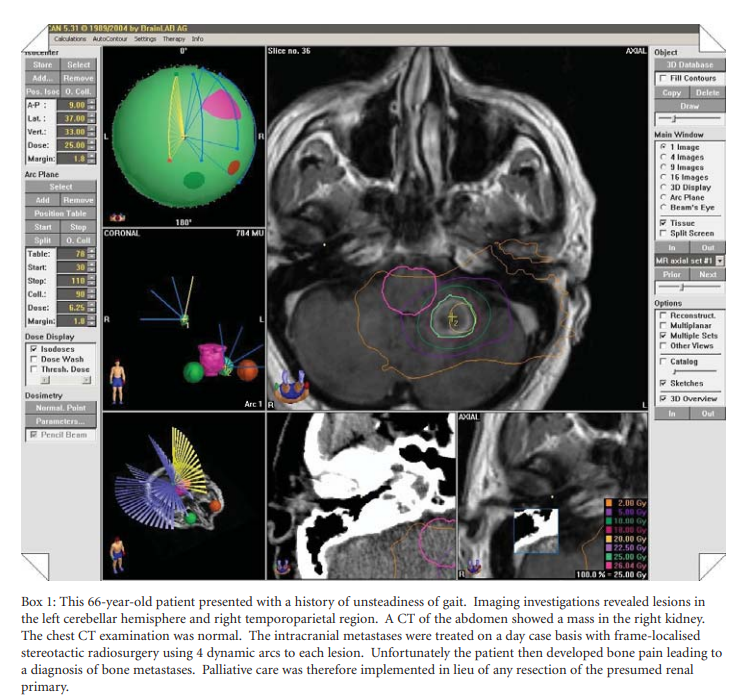
The literature is replete with case studies showing that SRS can provide local control of cerebral metastases that rivals the outcome of surgery + WBRT +/- tumour bed boost [14,15]. Such studies provide evidence to support the use of SRS but do not relegate surgery as a front-line therapy. For metastatic tumours in inaccessible and radiosensitive locations such as the brainstem, SRS does seem to confer significant survival and quality of life benefit [16]. However, class I evidence is lacking since a RCT comparing SRS versus surgical excision as the initial treatment for solitary intracranial metastases has not been performed. A clinical example of SRS treatment is provided in Box 1.
The role of SRS without WBRT for intracranial metastases
Recently debate has arisen regarding the use of SRS without adjuvant WBRT as a primary treatment for cerebral metastases. In a non-randomised comparative study 268 patients received SRS alone and 301 had SRS + WBRT. The median survivals between different paired prognostic groups were similar. However, 24% of the SRS alone group had salvage WBRT at disease relapse diluting the potential to demonstrate that WBRT confers outcome advantage [17].
A prospective randomised Japanese study has reported in abstract form that up-front WBRT (n=59) conferred significant benefit compared with SRS alone (n=61) in terms of local control and lowering the risk of developing further intracranial lesions although the median survival times between the treatment arms was similar [18]. If SRS alone is used, judicious posttreatment follow-up is required to detect new metastases or local failure enabling further SRS (to a new lesion) and/or WBRT to be administered.
Tumour Histology: Radiosensitivity
Melanoma, renal cell and non-small cell lung cancer metastases are relatively resistant to conventional fractionated radiotherapy. However, prospective and retrospective studies do indicate that SRS appears to be effective in achieving local control for these tumour types. It is feasible that a boost of SRS after WBRT has a more significant effect on increasing local control in this group than in patients with more radiosensitive tumours e.g. small cell cancer of lung, breast cancer.
Prognostic factors: Recursive Partitioning Analysis (RPA)
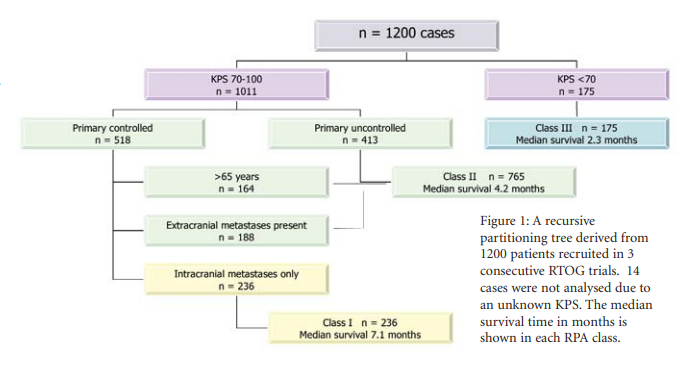
Recursive partitioning analysis is a regression tree analysis tool that has been used to define prognostic factors in patients with metastatic disease. In a cumulative series of 1200 RTOG trial recruits, Gaspar et al tested 21 variables for prognostic predictive value. The most effective discriminators of survival were the performance status (KPS 70 or greater vs. < 70); age (<65 vs. 65 or greater) and absence of systemic metastases. Using these nodal partitions the patients were categorised into three classes which correlated well with median survival times as shown in Figure 1 [19].
Chemotherapy and hormonal therapy in the management of patients with intracranial metastatic disease
Due to the differing sensitivity of tumour types and the complex combinations of surgery, WBRT and SRS, effective chemotherapeutic trials are difficult to conduct. At present mono or combination chemotherapy has not been shown to improve survival in patients with intracranial metastases. However, for tumours that are sensitive to chemotherapy and hormonal therapy, particularly breast cancer, these treatments are likely to maintain disease control outside the brain making effective control of intracranial disease important. Chemotherapy is sometimes adopted as a salvage treatment in a young patient with disease relapse. It appears that patients with small cell lung cancer and breast cancer may gain benefit from treatment regimes at this stage. In addition, evidence that ‘up front’, pre-irradiation chemotherapy may be an effective approach has been published. Randomised controlled trials are warranted in this area.
Newer treatments
Historically, most chemotherapeutic agents do not cross the blood brain barrier. However with the development of small molecules eg lapatinib (which is active against HER1 and HER2), this problem is likely to be overcome. Early trials suggest that treatment with lapatinib reduces the likelihood of patients with metastatic breast cancer developing cerebral metastases [20].
Clinical algorithm
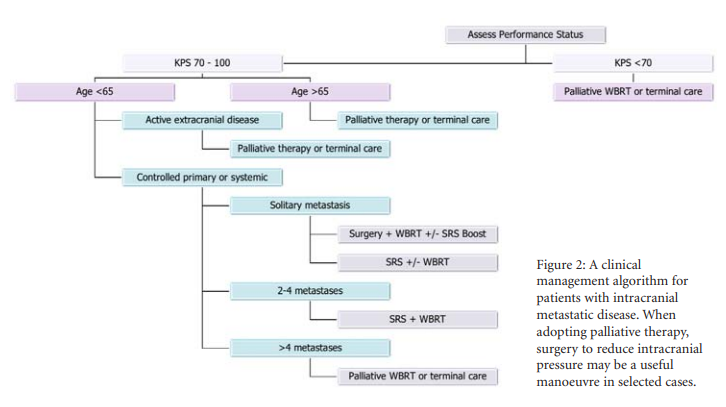
With improvements in systemic cancer treatment it is probable that increasing numbers of patients with intracranial metastases will warrant treatment by the neuro-oncology team. Best management requires a multidisciplinary approach with access to all treatment modalities including surgery, WBRT and SRS. The use of an evidence based recursive partitioning analysis guided clinical algorithm (see Figure 2) is of value in guiding individual patient therapy.
References
- Counsell C, Grant R. Incidence studies of primary and secondary intracranial tumours: a systematic review of their methodology and results. J Neuro Onc. 1998;37:241-50.
- Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases: histology, multiplicity, surgery and survival. Cancer. 1996;78:1781-8. https://doi.org/10.1002/(SICI)1097-0142(19961015)78:8<1781::AID-CNCR19>3.3.CO;2-Z
- Patchell RA, Tibbs PA, Walsh JW et al. A randomized trial of surgery in the treatment of single metastasis to the brain. N Engl J Med. 1990;322:494-500. https://doi.org/10.1056/NEJM199002223220802
- Cairncross JG, Kim J-H, Posner JB. Radiation therapy for brain metastases. Ann Neurol 1980;7:529-41. https://doi.org/10.1002/ana.410070606
- Richards P, McKissock W. Intracranial metastases. Br Med J. 1963;5322:15-8.
- Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48:384-94. https://doi.org/10.1002/1097-0142(19810715)48:2<384::AID-CNCR2820480227>3.0.CO;2-8
- Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer 1954;7:682-9. https://doi.org/10.1002/1097-0142(195407)7:4<682::AID-CNCR2820070409>3.0.CO;2-S
- Hart MG, Grant R, Walker M, Dickinson H. Surgical resection and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases (review). The Cochrane Database of Systematic Reviews. 2004: Issue 4.
- Vecht CJ, Haaxma-Reiche H, Noordijk EM et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583-90.
- Mintz AH, Kestle J, Rathbone MP et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78:1470-6.
- Patchell RA, Tibbs PA, Regine WF et al. Postoperative radiotherapy in the treatment of single metastases to the brain. A randomized study. JAMA. 1998;280:1485-9.
- Mintz AP, Caincross JG. Comment on: Treatment of a single brain metastasis – the role of radiation following surgical resection. JAMA. 1998; 280: 1527-9. https://doi.org/10.1001/jama.280.24.2128-JBK1223-2-1
- Andrews DW, Scott CB, Sperduto PW et al. Whole brain radiation therapy with or without stereotactic boost for patients with 1 to 3 metastases: Phase III results of the RTOG 9508 randomised trial. Lance.t 2005;63:1665-72.
- Muacevic A, Kreth FW, Horstmann GA et al. Surgery and radiotherapy compared with gamma knife radiosurgery in the treatment of solitary brain metastases of small diameter. J Neurosurg. 1999;91:35-43. https://doi.org/10.3171/jns.1999.91.1.0035
- Sheehan JP, Sun M-H, Kondziolka D et al. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long term outcomes and prognostic factors influencing patient survival time and local tumour control. J Neurosurg. 2002;97:1276-81.
- Huang C-F, Kondziolka D, Flickinger JC et al. Stereotactic radiosurgery for brainstem metastases. J Neurosurg. 1999;91:563-8. https://doi.org/10.3171/jns.1999.91.4.0563
- Sneed PK, Suh JH, Goetsch SN et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53:519-26.
- Aoyama H, Shirato H, Nakagawa K et al. Interim report of the JROSG99-1 multi-institutional randomized trial, comparing radiosurgery alone vs. radiosurgery plus whole brain irradiation for 1-4 brain metastases. J Clin Onc. 2004:22;108S, Abstract 1506.
- Gaspar L, Scott C, Rotman M et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51.
- Lin NU, Carey LA, Liu MC et al. Phase II trial of lapatinib for brain metastases in patients with HER2+ breast cancer. J Clin Onc 2006;2418S, Abstract 503.

