
An introduction to this series by Manoj Sivan can be found here.
Abstract
Chronic pain is a major cause of disability and healthcare burden worldwide. Despite this, there are currently few medications available to manage chronic pain, due to poor understanding of the underlying mechanisms. Studies in the current literature suggest that the brain alpha rhythm may be involved in pain perception. There is an inverse association between alpha activity and the perception of acute and chronic pain, which applies to alpha power in frontal and central brain regions specifically. As Alpha activity increases in frontal and central regions, pain perception decreases. Conversely increased attention to pain or expectation of pain suppresses alpha activity and increases pain perception. There is nascent evidence that increased alpha activity by external stimuli reduces the perception of experimental pain. Future research should investigate the potential of such novel treatments to reduce clinical pain.
Introduction
Chronic pain is one of the most common causes of disability worldwide.1 It is estimated that around 20% of adults in Europe suffer chronic pain with a large disease burden due to loss of productivity and difficulty in successful rehabilitation of these individuals.2 Despite these issues, the mechanisms underlying chronic pain are poorly understood and effective treatments are lacking. Indeed, evidence for the effectiveness of commonly used non-steroidal anti-inflammatories (NSAIDs) and opioids is limited. Furthermore, long-term use of opioids as would be required in the treatment of chronic pain results in dependency. A greater understanding of pain physiology, particularly the central mechanisms involved, is therefore required to improve pain management.
The alpha rhythm is one of the main EEG rhythms and represents oscillations ranging from 8-13Hz. Traditionally, alpha was thought of as an “idling” rhythm as high alpha power is associated with relaxed wakefulness. However, a more active role of alpha activity in neural processing has recently been proposed. Alpha is actively involved in modulation of sensory processing via a mechanism of functional inhibition and is thought to reflect top down control or attentional suppression.3 Interestingly, studies investigating EEG responses to pain suggest the alpha rhythm may play an active role in the processing of pain.
Alpha activity reduces in acute experimental pain
There is evidence to suggest pain decreases alpha activity through a process known as event-related desynchronisation (ERD), whereas pain relief increases alpha activity through a process known as event-related synchronisation (ERS). For example, Ohara et al. found in 4 healthy subjects that alpha ERD occurred as a response to transient noxious laser stimulation in several pain related brain regions including the primary somatosensory cortex and medial frontal cortex.4 Chang et al. found that the alpha rhythm is supressed following muscle pain but enhanced again upon waning pain in 15 healthy subjects.5 These studies suggest an inverse relationship between changes in alpha activity in response to a painful stimulus and the perception of pain, particularly in somatosensory and frontal brain regions.
Enhancing alpha activity before the onset of pain reduces pain intensity
Babiloni et al. investigated the effect of pre-stimulus alpha during the anticipation of pain on the intensity of experimental pain subsequently experienced in healthy individuals (n=10).6 A statistically significant negative correlation between pre-stimulus somatosensory alpha and pain intensity ratings was found during the early anticipation period (1 to 0.5s before pain stimulus onset). Tu et al. in a study involving 96 healthy individuals found a significant negative association between alpha power, specifically in the central brain regions and primary sensorimotor cortex, 0.2 to 0.03 seconds before the onset of pain and the intensity of pain subsequently experienced.7
Alpha activity is influenced by attention and expectations about pain
May et al. investigated the effects of attention on alpha power prior to the onset of noxious stimulation.8 They found that attending to the arm when pain is expected imminently suppresses alpha power in the contralateral primary somatosensory cortex and. Ploner et al. found that transient pain results in bilateral suppression of alpha activity in sensorimotor centres.9 They hypothesised that this occurs to “open the pain gate” and draw attention to pain so that an appropriate response can occur. Ohara et al. and Peng et al. found that attending to noxious stimulation results in a larger alpha ERD and perceived pain whereas distraction results in a lower alpha ERD and a subsequent analgesic effect.4,10 The above studies demonstrate that attention to pain supresses the alpha rhythm and increases pain perception, whereas distraction from the pain maintains the alpha rhythm and reduces pain perception.
Another factor that might influence the relationship between alpha and pain is expectations about pain. Our research group used a placebo experiment to investigate how expectation of pain relief can influence resting state alpha power in 73 healthy individuals (n=73).11 The study involved providing a placebo cream and testing the alpha power when subjected to a painful stimulus of the same intensity before and after application of the cream. There was an increase in alpha power accompanied by reduced pain perception suggesting a modulation of alpha power by reduced expectations of pain. Source localisation estimated that the increase of alpha originated from more frontal components of the pain network (and not the somatosensory area). These findings provide correlative evidence that frontal alpha is actively involved in the top-down control of pain via expectations about pain.
Low baseline alpha activity in chronic pain patients
A number of studies have demonstrated that alpha activity is reduced in chronic pain patients compared to healthy controls. Camfferman et al. investigated the correlation between alpha activity and pain intensity in a much more diverse sample of 103 patients with chronic pain.12 They found that alpha activity was inversely associated with pain intensity at frontal and central electrodes. Jensen et al. found that the proportion of alpha activity was significantly lower in patients with SCI and pain (n=38) compared to both SCI patients without pain (n=16) and healthy controls (n=28).13 They however found that absolute alpha activity in three frontal electrodes was positively correlated with pain intensity recorded. The authors hypothesised that an increase in frontal alpha activity may occur to suppress pain and patients with the largest pain ratings therefore would likely exhibit high frontal alpha activity to suppress it. This would actually be in line with the hypotheses made by Huneke et al. suggesting that frontal alpha activity is involved in top down modulation of pain and highlights that alpha activity in frontal and somatosensory regions may play different roles.11
Alpha entrainment reduces the perception of acute experimental pain
Alpha entrainment is one of the ways in which a causal relationship between the alpha rhythm and pain can be established. This process involves increasing alpha activity by providing rhythmic visual, auditory or transcranial electrical stimulation at an alpha range frequency.14 This allows cortical neurons to synchronise with the frequency of external stimulation, hence increasing the activity of neural oscillations in the alpha band. Open loop alpha entrainment involves providing external stimulation at a pre-determined fixed frequency within the alpha band, such as 10Hz. Closed loop entrainment involves first capturing the peak frequency of the alpha band in each subject using EEG, known as the individual alpha frequency (IAF), and then providing external stimulation at this personalised frequency.
Ecsy et al. from our research group investigated whether auditory entrainment (using binaural beats) or visual alpha entrainment (rhythmic visual stimulation) could increase alpha activity and reduce the perception of experimental acute pain in 64 healthy individuals.15,16 The study involved entraining at 8 Hz, 10 Hz and 12 Hz, we found that entrainment using 10Hz visual stimulation resulted in the greatest analgesic effect, reducing pain by 1.1 on the 10-point scale. Whilst this may not be regarded as a large clinically significant effect, it does demonstrate a proof of concept that alpha entrainment can reduce pain perception
Alpha entrainment reduces clinical pain
Arendsen et al. used a form of transcranial electrical stimulation known as transcranial alternating current stimulation (tACS) to attempt to modulate the perception of experimental pain in healthy individuals in conditions where the intensity of upcoming noxious stimulation was certain or uncertain (n=23).17 This crossover trial involved participants receiving 10 Hz somatosensory tACS or sham stimulation on separate days with a 7-day washout period in between. The study found a significant reduction in perceived pain intensity and unpleasantness during the alpha tACS compared to sham stimulation, but only when the intensity of upcoming pressure stimulation was uncertain. In a more recent crossover sham-controlled study, 20 participants with chronic lower back pain (CLBP) who received 40 minutes of alpha tACS were able to significantly increase alpha activity in somatosensory regions and decrease pain score compared to sham control stimulation.18 Our recent study on visual alpha entrainment in individuals with chronic pain shows analgesic effect in some individuals with chronic pain.19 These findings are of great interest as they provide strong causal evidence that the alpha rhythm reduces the perception of chronic pain and indicate a possible dose-effect of increased alpha activity on reduced pain perception.
Neurofeedback for alpha entrainment in chronic pain
Jensen et al. investigated whether individuals actively increasing alpha power using neurofeedback techniques could reduce chronic pain.20 The process involves subjects receiving biofeedback on their own EEG and learning how to enhance certain rhythms. They found chronic pain perception was significantly reduced from baseline to directly after the intervention of increasing their alpha power. There were no significant changes in the quality of sleep, fatigue or pain interference, suggesting the alpha rhythm may modulate experience of pain independently of these other factors. Our recent systematic review and meta-analysis concluded neurofeedback to be a safe and effective therapy in chronic pain.21
Discussion
Current evidence suggests the alpha rhythm is a negative modulator of pain perception and is itself negatively modulated by noxious stimulation, attention and pain expectancy. A model for the role of the alpha rhythm in the perception and modulation of pain in conceptualised in Figure 1.
Figure 1. Proposed role of the alpha rhythm in the perception and modulation of pain
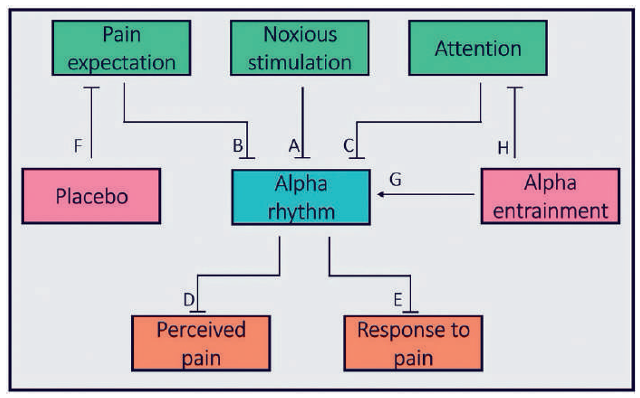
Fig 1: Various factors involved in the perception of pain are given and the interactions between each are denoted by either straight headed arrows denoting suppression or arrow headed arrows denoting stimulation.
Key:
A – Noxious stimulation supresses the alpha rhythm.4
B – Expectation of pain supresses the alpha rhythm.6
C – Attention to pain supresses the alpha rhythm.13
D – The alpha rhythm supresses the perception of pain.16,17
E – The alpha rhythm supresses the wider ability to form an appropriate response to pain.9
F – Placebo supresses the expectation of pain.11
G – Alpha entrainment increases alpha power.16
H – Alpha entrainment may cause distraction and hence suppress attention to pain.
Whilst the emerging causal evidence for the role of alpha in pain is promising, the small sample sizes and lack of a control group in some studies limit the confidence in the conclusions that can be made. Larger sham-controlled studies are required to investigate whether alpha entrainment and neurofeedback can increase alpha activity and decrease perceived pain. Alpha modulatory studies into chronic pain should also include participants with a variety of common painful conditions, such as osteoarthritis, chronic widespread pain and fibromyalgia. Our current understanding of neurophysiology supports common central mechanisms in chronic pain irrespective of the pathological diagnosis.
Further studies are also required to establish if there is a dose-response effect of enhancing alpha activity on pain relief. Future studies on alpha entrainment and neurofeedback should explore whether such therapy can be delivered in “doses” similar to pharmacological interventions. There is also a possibility of that those with a greater degree of central sensitisation may respond better to entrainment. An understanding of this will help personalise such novel treatments based on symptoms and optimal dose of entrainment for every individual. We have recently shown the usability and acceptability of smartphone-based applications for alpha entrainment in individuals with chronic pain.22 We also have ongoing clinical studies involving the use of neurofeedback to increase alpha activity and decrease chronic pain in our research group.
If the underlying physiological role of the alpha rhythm in chronic pain is confirmed, there will be a requirement for large clinical randomised controlled trials to assess the efficacy, optimal dose, duration of the therapeutic effect of each dose, and subsequently what the optimum combinations of open or closed loop entrainment or neurofeedback might be and how these interact with more conventional therapies. Clinical acceptability studies with patients should also be performed to determine whether potential therapies could be tolerated, whether there are any side effects, and how best to deliver modulation of alpha activity in the home setting, through alpha entrainment or neurofeedback.
Conclusions
In summary, studies suggest the alpha rhythm may act as a top-down negative modulator of pain and is itself negatively modulated by attention and pain expectancy. This suggests the alpha rhythm is a promising target for non-pharmacological pain management strategies. However, further causal evidence is required to better establish the relationship between alpha and pain and to determine whether the treatment strategies of entrainment and neurofeedback could be clinically effective and individualised.
References
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. doi:10.1016/S0140-6736(12)61729-2
- van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth. 2013;111(1):13-18. doi:10.1093/bja/aet123
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi:10.3389/fnhum.2010.00186
- Ohara S, Crone N., Weiss N, Lenz F. Attention to a painful cutaneous laser stimulus modulates electrocorticographic event-related desynchronization in humans. Clin Neurophysiol. 2004;115(7):1641-1652. doi:10.1016/J.CLINPH.2004.02.023
- Chang P, Arendt-Nielsen L, Graven-Nielsen T, Svensson P, Chen A. Different EEG topographic effects of painful and non-painful intramuscular stimulation in man. Exp Brain Res. 2001;141(2):195-203. doi:10.1007/s002210100864
- Babiloni C, Brancucci A, Percio C Del, et al. Anticipatory Electroencephalography Alpha Rhythm Predicts Subjective Perception of Pain Intensity. J Pain. 2006;7(10):709-717. doi:10.1016/J.JPAIN.2006.03.005
- Tu Y, Zhang Z, Tan A, et al. Alpha and gamma oscillation amplitudes synergistically predict the perception of forthcoming nociceptive stimuli. Hum Brain Mapp. 2016;37(2):501-514. doi:10.1002/hbm.23048
- May ES, Butz M, Kahlbrock N, Hoogenboom N, Brenner M, Schnitzler A. Pre- and post-stimulus alpha activity shows differential modulation with spatial attention during the processing of pain. Neuroimage. 2012;62(3):1965-1974. doi:10.1016/j.neuroimage.2012.05.071
- Ploner M, Gross J, Timmermann L, Pollok B, Schnitzler A. Pain Suppresses Spontaneous Brain Rhythms. Cereb Cortex. 2006;16(4):537-540. doi:10.1093/cercor/bhj001
- Peng W, Hu L, Zhang Z, Hu Y. Changes of spontaneous oscillatory activity to tonic heat pain. PLoS One. 2014;9(3):e91052. doi:10.1371/journal.pone.0091052
- Huneke NTM, Brown CA, Burford E, et al. Experimental placebo analgesia changes resting-state alpha oscillations. PLoS One. 2013;8(10):e78278. doi:10.1371/journal.pone.0078278
- Camfferman D, Moseley GL, Gertz K, Pettet MW, Jensen MP. Waking EEG Cortical Markers of Chronic Pain and Sleepiness. Pain Med. 2017;18(10):1921-1931. doi:10.1093/pm/pnw294
- Jensen MP, Sherlin LH, Gertz KJ, et al. Brain EEG activity correlates of chronic pain in persons with spinal cord injury: clinical implications. Spinal Cord. 2013;51(1):55-58. doi:10.1038/sc.2012.84
- Thut G, Schyns PG, Gross J. Entrainment of Perceptually Relevant Brain Oscillations by Non-Invasive Rhythmic Stimulation of the Human Brain. Front Psychol. 2011;2:170. doi:10.3389/fpsyg.2011.00170
- Ecsy K, Jones AKP, Brown CA. Alpha-range visual and auditory stimulation reduces the perception of pain. Eur J Pain. 2017;21(3):562-572. doi:10.1002/ejp.960
- Ecsy K, Brown CA, Jones AKP. Cortical nociceptive processes are reduced by visual alpha-band entrainment in the human brain. Eur J Pain. 2018;22(3):538-550. doi:10.1002/ejp.1136
- Arendsen LJ, Hugh-Jones S, Lloyd DM. Transcranial Alternating Current Stimulation at Alpha Frequency Reduces Pain When the Intensity of Pain is Uncertain. J Pain. 2018;19(7):807-818. doi:10.1016/j.jpain.2018.02.014
- Ahn S, Prim JH, Alexander ML, McCulloch KL, Fröhlich F. Identifying and Engaging Neuronal Oscillations by Transcranial Alternating Current Stimulation in Patients With Chronic Low Back Pain: A Randomized, Crossover, Double-Blind, Sham-Controlled Pilot Study. J Pain. 2019;20(3):277.e1-277.e11. doi:10.1016/j.jpain.2018.09.004
- Arendsen LJ, Henshaw J, Brown CA, Sivan M, Taylor JR, Trujillo-Barreto NJ, Casson AJ, Jones AKP Entraining alpha activity using visual stimulation in patients with chronic musculoskeletal pain. A feasibility study. Frontiers in Neuroscience (accepted) https://www.frontiersin.org/articles/10.3389/fnins.2020.00828/abstract
- Jensen MP, Gertz KJ, Kupper AE, et al. Steps Toward Developing an EEG Biofeedback Treatment for Chronic Pain. Appl Psychophysiol Biofeedback. 2013;38(2):101-108. doi:10.1007/s10484-013-9214-9
- Patel K, Sutherland H, Henshaw J, Taylor JR, Brown CA, Casson AJ, Trujillo-Barreton NJ, Jones AKP, Sivan M. Effects of neurofeedback in the management of chronic pain: A systematic review and meta-analysis of clinical trials. Eur J Pain. 2020 Jun 5
- Locke HN, Brooks J, Arendsen LJ, Jacob NK, Casson A, Jones AKP, Sivan M. Acceptability and usability of smartphone-based brainwave entrainment technology used by individuals with chronic pain in a home setting. British Journal of Pain. Published online first Feb 21, 2020.


