Abstract
Myasthenia gravis (MG) is an autoimmune disease of the neuromuscular junction. Whilst a number of immune therapies have been established in recent decades, there is an ongoing need for safe and effective therapies, for treatment refractory patients. A number of recent trials have studied the effect of modern immunotherapies, targeting circulating immune cells, complement and Fc receptor inhibition. We will review these agents and the data relating to their use in MG.
Key take-home messages
- A number of agents, including several monoclonal antibodies using new mechanisms of action, have shown promising results in MG in recent studies.
- Therapies targeting B cells, such as rituximab, have become more established, although adequate randomised controlled trial data is lacking. Newer anti-CD19/20 therapies may offer opportunities for future trials.
- Complement inhibition, notably with eculizumab, has progressed to FDA listing for MG treatment after phase 3 trials.
- FcR inhibitors, including efgartigimod and rozanolixuzumab, that decrease pathogenic IgG have shown positive results in phase 2 trials.
Myasthenia gravis (MG) is an uncommon disorder of immune mediated dysfunction of the neuromuscular junction, typically presenting with fatigable muscle weakness. Around 80% of cases are positive for the acetylcholine receptor (AChR) antibody, with the remainder made up of less common antibodies including muscle-specific kinase (MuSK), lipoprotein receptor-relatedprotein-4 (LRP4), and so-called seronegative cases. Treatment for myasthenia gravis includes inhibition of acetylcholine breakdown with acetylcholinesterase inhibitors, corticosteroids, a range of immunosuppressive steroid sparing agents and immunomodulatory therapy such as intravenous immunoglobulin (IVIg), plasma exchange and thymectomy.1 Performing randomised trials in MG with a positive outcome has proven repeatedly challenging in the last decades, because of the rarity of the disease, the complexity of treatment avenues and the heterogeneity of clinical presentations. Even agents widely accepted to be beneficial in MG, such as mycophenolate or rituximab, were not found to be superior to placebo in phase 2 and 3 trials.2 Of note, thymectomy for generalised AChR positive MG has shown promising evidence over a three-year period for reducing corticosteroid requirements, improvement in clinical scoring tools and reducing hospitalisations.3
Improved understanding of the underlying immunology of MG has led to a number of new agents being studied in recent years. These agents have targeted biological features of MG, including the role of T helper cells promoting B cell maturation and antibody production, AChR antibodies activating complement, and pathogenic IgG subclasses.4 Arising evidence for the usage of these novel agents in MG will be reviewed here.
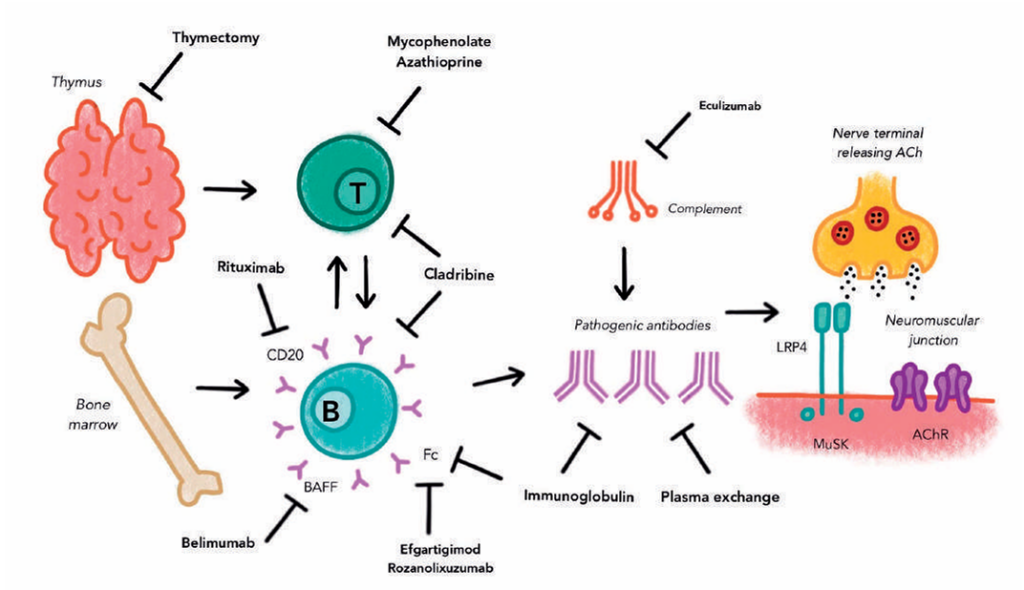
B Cell directed therapy
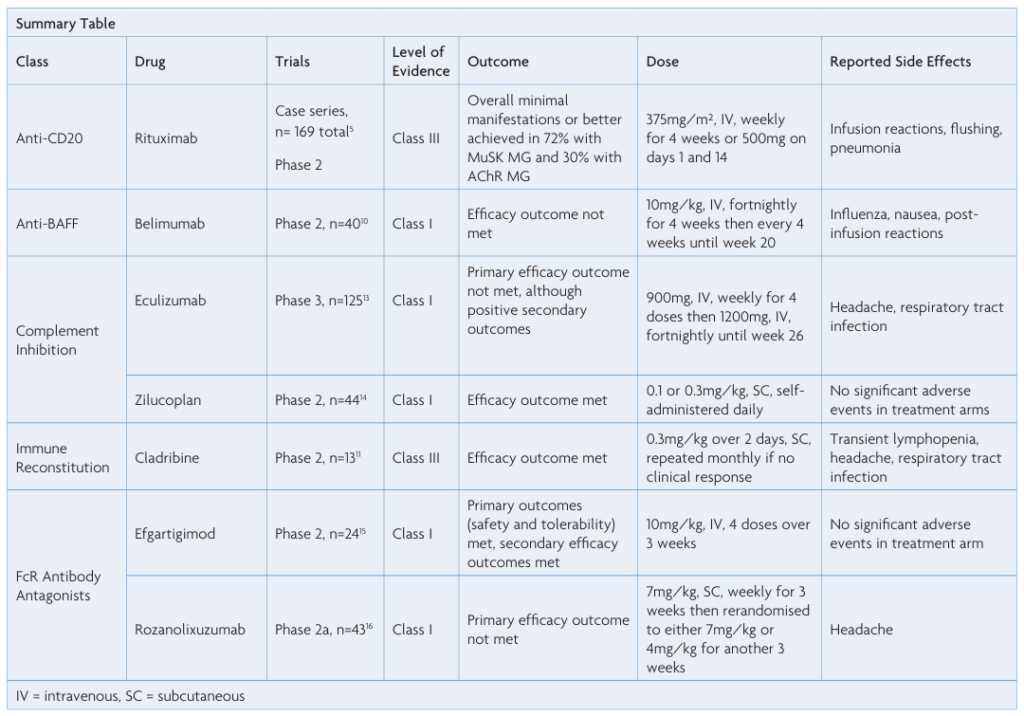
Rituximab (RTX) is an intravenously administered monoclonal antibody which binds CD20 and causes depletion of circulating B cells. It is used for treatment of lymphomas as well as a range of autoimmune diseases, including MG. A number of retrospective case series have reported short-term and long-term efficacy in patients with AChR and MuSK positive MG, whilst maintaining an acceptable side-effect profile.5-7 Described adverse effects have included infusion reactions and flushing, with infections occurring uncommonly.5 In contrast, a phase 2 randomised controlled trial (RCT) of RTX in AChR positive MG patients was negative (Beat MG, presented AANEM 2019, unpublished). At present, RTX is commonly used for treatment of refractory MG, with a widespread perception that MuSK positive patients respond particularly well.8 A more formal analysis based on a sufficiently powered RCT is needed.
Several new monoclonal antibodies targeting CD19 and CD20 are an area of interest for treatment of MG, but are yet to enter clinical trials. Obinutuzumab, a novel anti-CD20 monoclonal antibody, was reported to induce remission of MG in a patient co-treated for chronic lymphocytic leukaemia.9
Another avenue of B cell directed therapy is B cell activation factor (BAFF), a cytokine important in survival and differentiation of B cells. A number of agents targeting BAFF, such as belimumab, have been trialled in other autoimmune conditions, such as lupus erythematosus.
Identification of increased BAFF levels in MG lead to examination for potential treatment efficacy. A phase 2, double-blind, placebo-controlled study randomising 40 patients to intravenous belimumab 10mg/kg and placebo did not show any statistical significance in the Quantitative Myasthenia Gravis (QMG) score, the primary outcome for this study.10 Adverse events included influenza, nausea and post-infusionreactions.10
Cladribine
Cladribine is a deoxyadenosine analogue which induces a rapid and selective B and T lymphocyte toxicity through accumulation of 2-chlorodeoxyadenosine nucleotides. It has established indications in hairy cell leukaemia and multiple sclerosis.
The use of cladribine in myasthenia gravis was examined in a recent prospective open label study in Poland.11 Thirteen patients with seropositive generalised and ocular MG were enrolled, and immunosuppressant therapy other than prednisolone was stopped prior to receiving cladribine. Cladribine was administered subcutaneously at 0.3mg/kg divided into two doses over two days. This was repeated after one month, and one patient had another dose at month three. The primary outcome of Improvement in the Myasthenia Gravis Composite (MGC) scale of at least three points after six months was met in 11 of the 13 patients. Eleven patients were on prednisolone when cladribine was started, after six months eight of those had ceased prednisolone. Transient lymphopenia was reported.11 Limitations of the study included the absence of a control population and blinding.
The role of immune reconstitution therapies in MG requires further investigation.
Complement Inhibition
Complement plays an important role in disease pathogenesis in myasthenia gravis. Patients with MG demonstrate the presence of C3 and the membrane attack complex at the NMJ and complement inhibition has been associated with decreased disease severity in animal models of MG.12 Complement causes inflammation and mediates damage to the postsynaptic NMJ membrane.13
Eculizumab is a monoclonal antibody designed to bind to human terminal complement C5, thereby inhibiting mediation of proinflammatory cell chemotaxis by C5a and formation of the membrane attack complex by C5b.13 Eculizumab is used in complement mediated diseases, such as paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome.
A phase 3 RCT, REGAIN, examined the effect of eculizumab in 125 patients with refractory generalised MG and seropositive AChR status. Eculizumab was administered intravenously each week for four weeks then fortnightly to complete 26 weeks. Primary outcome was measured by change in the MG-ADL, another validated scoring tool designed to assess MG severity, after 26 weeks. Statistical significance was not reached for the primary endpoint; however, there was measured improvement in secondary outcomes, including hospitalisations and exacerbations. Vaccination against Neisseria meningitides was a mandatory inclusion criterion due to the increased risk of infection secondary to complement inhibition.13
On the basis of promising trial results, eculizumab was the first biologic agent to be FDA approved for MG, although its place in therapy relative to cost remains to be seen.
Zilucoplan is a macrocytic peptide which binds C5 and prevents cleavage into C5a and C5b.14 Zilucoplan given by daily subcutaneous injection was studied in a phase 2 trial, with double-blind randomisation of 45 patients between doses of 0.1mg/kg, 0.3mg/kg and placebo for a period of 12 weeks. Differences in QMG score were recorded in both the 0.1mg/kg and 0.3mg/kg arms compared with placebo with a mean improvement of 2.8 points in 0.3mg/kg arm. No significant adverse events were described in the trial.14
In summary, complement inhibition appears to be modestly beneficial in patients with refractory MG; the use of these agents is impacted by the risk of bacterial infections and high costs of some of these agents.
FcR Antibody Antagonists
Depletion of pathogenic circulating immunoglobulins, usually with plasma exchange, is an established treatment modality for immunologic disorders. More recently, molecules specifically antagonising the neonatal Fc receptor to rapidly reduce circulating IgG levels have been examined.
Efgartigimod is an anti-neonatal Fc receptor immunoglobulin fragment. A double-blind, placebo-controlled, phase 2 RCT randomised12 patients with seropositive AChR MG to receive intravenous efgartigimod and 12 to placebo for a period of three weeks.15 Rapid and sustained reduction in all IgG subtypes was observed, including levels of the AChR antibody levels, which persisted for around 29 days. Secondary outcomes included clinical measures, which demonstrated an improvement in the QMG score after the first dose and MG-ADL at 29 and 36 days. No significant adverse events were noted during the study.15
A second FcR antibody antagonist, rozanolixizumab, demonstrated proof-of-concept in a recent phase 2a trial.16 Twenty-one patients received weekly subcutaneous rozanolixizumab and 22 received placebo prior to re-randomisation at day 29 to either 7mg/kg or 4mg/kg of rozanolixizumab. The primary outcome was not reached, with non-significant difference in QMG score between rozanolixizumab and placebo groups. Rozanolixizumab has progressed to phase 3 trials which are ongoing (NCT03971422). The drug appeared well tolerated, although headache was reported.16
Conclusion
Performing RCTs in MG has proven difficult in the last decades, due to the rarity of this illness, clinical and biological heterogeneity and the numerous overlapping therapies in common usage. Relatively short durations of clinical trials may understate treatment effect and reduce the likelihood of significant findings. Lack of standardisation of clinical scoring tools can hamper comparison between studies. Remaining on standard therapy at trial commencement can create a floor effect, where already low MG severity scale scores fail to demonstrate statistically significant improvements. As such, it is encouraging to see renewed interest to study novel agents in this vulnerable patient group. The relative risk of additive immunosuppression in this group requires careful consideration and patient discussion and will benefit from greater understanding with further studies.
References
- Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I, et al. International consensus guidance for management of myasthenia gravis. Neurology. 2016;87(4):419-25. https://doi.org/10.1212/WNL.0000000000002790
- Sanders DB, Hart IK, Mantegazza R, Shukla SS, Siddiqi ZA, De Baets MHV, et al. An international,phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology. 2008;71(6):400-6. https://doi.org/10.1212/01.wnl.0000312374.95186.cc
- Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo H-C, Marx A, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. New England Journal of Medicine. 2016;375(6):511-22. https://doi.org/10.1056/NEJMoa1602489
- Evoli A. Myasthenia gravis. Current Opinion in Neurology. 2017;30(5):464-70. https://doi.org/10.1097/WCO.0000000000000473
- Tandan R, Hehir MK, Waheed W, Howard DB. Rituximab treatment of myasthenia gravis: A systematic review. Muscle & Nerve. 2017;56(2):185-96. https://doi.org/10.1002/mus.25597
- Chan F, Swayne A, Gillis D, Walsh M, Henderson RD, McCombe PA, et al. Long-term follow-up of patients with myasthenia gravis treated with low-dose rituximab. Journal of neurology, neurosurgery, and psychiatry. 2019;90(8):955-6. https://doi.org/10.1136/jnnp-2018-319410
- Beecher G, Anderson D, Siddiqi ZA. Rituximab in refractory myasthenia gravis: Extended prospective study results. Muscle Nerve. 2018;58(3):452-5. https://doi.org/10.1002/mus.26156
- Hehir MK, Hobson-Webb LD, Benatar M, Barnett C, Silvestri NJ, Howard JF, Jr., et al. Rituximab as treatment for anti-MuSK myasthenia gravis: Multicenter blinded prospective review. Neurology. 2017;89(10):1069-77. https://doi.org/10.1212/WNL.0000000000004341
- Russell A, Yaraskavitch M, Fok D, Chhibber S, Street L, Korngut L. Obinutuzumab Plus Chlorambucil in a Patient with Severe Myasthenia Gravis and Chronic Lymphocytic Leukemia. J Neuromuscul Dis. 2017;4(3):251-7. https://doi.org/10.3233/JND-170211
- Hewett K, Sanders DB, Grove RA, Broderick CL, Rudo TJ, Bassiri A, et al. Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis. Neurology. 2018;90(16):e1425-e34. https://doi.org/10.1212/WNL.0000000000005323
- Rejdak K, Szklener S, Korchut A, Baranowski D. Cladribine in myasthenia gravis: a pilot open‐label study. European Journal of Neurology. 2020;27(3):586-9. https://doi.org/10.1111/ene.14124
- Soltys J, Kusner LL, Young A, Richmonds C, Hatala D, Gong B, et al. Novel complement inhibitor limits severity of experimentally myasthenia gravis. Ann Neurol. 2009;65(1):67-75. https://doi.org/10.1002/ana.21536
- Howard JF, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. Safety and efficacy ofeculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. The Lancet Neurology. 2017;16(12):976-86. https://doi.org/10.1016/S1474-4422(17)30369-1
- Howard JF, Nowak RJ, Wolfe GI, Freimer ML, Vu TH, Hinton JL, et al. Clinical Effects of the Self-administered Subcutaneous Complement Inhibitor Zilucoplan in Patients With Moderate to Severe Generalized Myasthenia Gravis. JAMA Neurology. 2020. https://doi.org/10.1001/jamaneurol.2019.5125
- Howard JF, Bril V, Burns TM, Mantegazza R, Bilinska M, Szczudlik A, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92(23):e2661-e73. https://doi.org/10.1212/WNL.0000000000007600
- Bril V, Benatar M, Brock M, Greve B, Kiessling P, Woltering F, et al. Proof-of-Concept and Safety of the Anti-FcRn Antibody Rozanolixizumab in Patients with Moderate-to-Severe Generalized Myasthenia Gravis (GMG): A Phase 2a Study (S43.001). Neurology. 2019;92(15Supplement):S43.001.

