Abstract
Because of its gradually declining incidence, many UK physicians rarely encounter leprosy which is thus easily overlooked. Hansen’s discovery of mycobacterium leprae, the first identified human bacterial infection, was of crucial importance. He struggled to find acceptance, and arguments about the priority of his discovery were rife until his vital role was established in the Leprosy congress in Berlin in 1897.
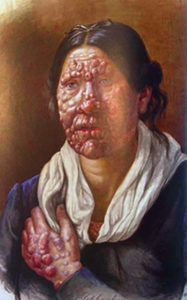
Leprosy was the first proven instance of bacterial infection causing human disease. Its importance cannot be over-emphasised, because it was the first direct evidence of the causal role of microorganisms predicted but not proved by the Germ theory of the 1800s that stated many diseases are caused by the presence and actions of specific microorganisms. Germ Theory superseded existing theories of disease: divine superstitions, miasma, and contagion, and thereby revolutionised the practice of Medicine. The global prevalence has decreased from approximately 600, 000 cases in 2001 to <200,000 in 2015 [1], but under-reporting is likely. It remains a worldwide scourge [2].
The existence of ‘germs’, ‘microbes’, or ‘microorganisms’ however, had preceded Germ Theory, for in 1676-7, using his first microscope, Antonie van Leeuwenhoek (1632–1723) of Delft, observed tiny organisms — which he called ‘animalcules’ — but he did not relate them to disease. It was Louis Pasteur (1822-95) and Robert Koch (1843-1910) who showed that microbes caused infectious diseases. Pasteur showed in 1863 that microorganisms caused the fermentation of milk and grapes, and the putrefaction of meat. He, therefore, suggested human disease was caused by the multiplication of germs in the body [3].
Koch proved that Bacillus anthracis that he observed in the tissue of anthrax victims was the cause of anthrax [4] and crucially was transmissible. He extracted this bacterium from a sheep that had died of anthrax, then repeatedly injected generations of mice with it; the mice developed the disease. Koch announced in 1876 that he had proved this bacterium caused anthrax. He went on to identify the bacterial causes of tuberculosis (1882), and cholera (1883).
Gerhard Armauer Hansen (1841-1912) worked at St. Jørgen’s hospital in Bergen, striving to find the cause of leprosy. Importantly, Hansen’s identification of M. leprae preceded Koch’s seminal investigations [5]. Leprosy is a chronic mycobacterial infection that affected millions of people, mainly in Brazil, India, and Indonesia.
The Tuberculoid or paucibacillary type shows well-expressed cell-mediated immunity that controls bacillary multiplication by forming epithelioid-cell granulomas. The lepromatous or multibacillary type shows cellular anergy towards M. leprae, thus profuse bacillary multiplication. Between these is a continuum, varying from borderline tuberculoid, through borderline, to those with little cellular response, borderline lepromatous.
Clinical Features
Hansen had observed varied clinical features. Typically there was a variety of skin lesions: a large single well-defined red patch or asymmetrical hypo-pigmented macules, plaques, and nodules, often thickening of the forehead and earlobes, loss of eyebrows, deformity of the nose, and loss of the upper incisor teeth. Neuropathy was accompanied by sensory loss and tender, thickened peripheral nerves. It could resolve spontaneously or progress to borderline or lepromatous leprosy with hideous deformities.
Hansen also observed visual impairment or blindness now known to result either from mycobacterial infiltration of the anterior segment of the eye or from trophic changes caused by damage to trigeminal and facial nerves, resulting in lagophthalmos, deformed eyelids, or corneal anaesthesia.
Hansen’s bacillus

Hansen was born to a Danish family the eighth of 15 children, at Bergen on July 29, 1841. His father Claus was a merchant who after a crisis went bankrupt. His memoirs show details of his childhood and professional life [6]. He studied Medicine at the University of Christiania (now Oslo), qualifying in 1866. In 1870, he went to Bonn and Vienna to study histopathology. Returning to Bergen in 1868, he was appointed assistant physician to the distinguished Daniel Cornelius Danielssen (1815-1894) at St Jørgen’s hospital for lepers. He searched for the aetiology of leprosy, in blood, then in leprous tissues. It was here that Hansen discovered the causative agent of leprosy, M. lepra, in 1873 [7,8,9]. This was three years before Koch identified the anthrax bacillus and nine years before he showed that tubercle bacillus caused human tuberculosis.
The prevalence of leprosy in Bergen was high, estimated at c. 25 per 1,000 population. With Carl Wilhelm Boëck (1808-1875) of Oslo, Danielssen had pioneered the scientific study of leprosy [10]. St Jørgen’s hospital was established in the 14th-century and became a famous leprosy research centre. Danielssen believed leprosy was hereditary, but Hansen suspected it was infectious.
Having observed yellowish granular masses in leprous tissues in 1869, in 1873 he discovered the rod shaped bodies that looked like bacteria in ‘lepromata’, later identified as M. Leprae. ‘Danielssen at first disputed Hansen’s evidence of an infectious cause. Hansen read a paper in 1874 to the Medical Society of Christiania, claiming these bodies were the cause of leprosy. It was published in the Norsk Magazin for Laegevid–enskaben, as an 88-page monograph in 1874. However, written in Norwegian, its readership was limited and failed to excite interest in the medical world. We can recognise his scientific caution, for at first, he was uncertain whether these rod-shaped bodies were bacteria, perhaps because he failed to culture or stain them adequately:
There are to be found in every leprous tubercle extirpated from a living individual — and I have examined a great number of them — small staff-like bodies, much resembling bacteria, lying within the cells; not in all, but in many of them. Though unable to discover any difference between these bodies and true bacteria, I will not venture to declare them to be actually identical. Further, while it seems evident that these low forms of organic life [i.e., bacteria] engender some of the most acute infectious diseases, the attributing of the origin of such a chronic disease as leprosy to the apparently same matter must, of course, be attended with still greater doubts. It is worthy of notice, however, that the large brown elements found in all leprous proliferations in advanced stages … bear a striking likeness to bacteria in certain stages of development…” [11]
But after consulting Robert Koch, he improved his techniques and in 1880 did stain the bacilli. However, in 1879, Albert Neisser (discoverer of Neisseria gonorrhoea) claimed discovery of the leprosy bacillus, using material he obtained from Hansen during a visit to Bergen. Using superior fuchsin and gentian violet stains, Neisser described the lepra bacilli, their appearance and distribution [12].
Danielssen however, encouraged Hansen to counter Neisser’s claim for priority [13]. An angered Hansen updated his original report to publish new articles simultaneously in English [11], German, and Norwegian journals in 1880, establishing his priority in the discovery [14,15].
I feel myself called upon to announce what I have attained to, up to the present time, in my researches after the same ‘contagium,’ and, this, partly to assert my priority with reference to this discovery, and partly in order to advance those details in research which I omitted to announce on account of the still uncertain result in my report to the Medical Society of Christiania, 1874, concerning my investigations into the aetiology of leprosy.”
However, Neisser later retracted his claim for priority:
I have never claimed for myself the priority of having been first to see bacteria in leprosy… [16]

Although Hansen had described acid, alcohol resistant bacilli in leprous lesions, he struggled in vain to culture M. Leprae or to satisfy Koch’s postulates*. Frustrated, in 1879, he inoculated the eye of a woman with material drawn from a leprous nodule. But because he had failed to obtain informed consent Hansen was found guilty of unethical conduct when tried at Court in Bergen. He lost his position as physician of the Bergen hospitals, but retained his job as leprosy medical officer for the entire country of Norway. Henry Vandyke Carter (a major contributor to Gray’s Anatomy, and leprologist in India) visited Hansen’s laboratory and vigorously supported him and his conclusions that leprosy was due to an infectious bacillus, in his article On leprosy and elephantiasis. (London: Eyre and Spottiswoode,1874). At a Leprosy congress in Berlin (1897) Hansen was finally recognised [17] as the discoverer of the lepra bacilli, the cause of leprosy. Leprosy is often named ‘Hansen’s disease.’
Hansen then worked at the Bergen Museum. He wrote extensively and sought to spread Darwin’s theory of evolution. He also wrote about whaling and marine life.
In 1873, he married Danielssen’s daughter, Stephanie Marie, who died of tuberculosis only months later. It is reported that he suffered with syphilis and died of ‘a heart attack’ in 1912, aged 71. Hansen was to receive many honours, including the doctorate of the University of Copenhagen. His statue was erected in the gardens of the Bergen Museum eleven years before his death on 13 February, 1912, at which time his ashes were placed in a bronze urn beneath the bust.
Origins of leprosy
The word lepra in Latin was applied to patients, but historically was known as Elephantiasis Grecorum, which in turn replaced the Greek lepra taken from the biblical word tsara’ath. It was often mistranslated as leprosy, though literally it signified any disease with a scaly skin, such as psoriasis, scabies, and syphilis. Before Hansen’s discovery, Robley Dunglison, personal physician to Thomas Jefferson, showed the bewildering variety of names in his textbook, The Practice of Medicine [18]:
II ELEPHANTIASIS GRECORUM SYNON. Lepra tuberculosa, L.Aegyptiaes,
- Alba, L. Hebaeorum, L. leontina,
- Mosaica, Tsarath of Moses, Leontiasis, Satyriasis, Lepra, Leuce, Morphaea alba, Baras, Vitiligo; Fr. Lèpre tuberculeuse, Elèphantiasis des Grecs, Ladrerie, Tete de veau, Mal rouge de Cayenne; Ger. Weisse oder Mosaiche Aussatz, Knolligre Aussatz.
In the bible, Lepers were ‘unclean’ and isolated from the community. Their plight is shown in Leviticus (13.2):
When a man has on the skin of his body a swelling or a scab or a bright spot, and it becomes an infection of leprosy on the skin of his body, then he shall be brought to Aaron the priest or to one of his sons the priests. … All the days wherein the plague shall be in him he shall be defiled; he is unclean: he shall dwell alone; without the camp shall his habitation be.
And in Matthew (8:3):
And Jesus put forth [his] hand, and touched him, saying, I will; be thou clean. And immediately his leprosy was cleansed.
Leprosaria
Although leprosy is now known not to be highly contagious, since biblical edicts lepers were stigmatised and condemned to a lifetime of monastic isolation, social abhorrence, fear and rejection. In AD 625, the first-known leper hospital in England, was founded at Nottingham (Blyth Leper Hospital). And in 1101 Queen Matilda, wife of Henry I, founded a leper house at St Giles in the Fields, London. Throughout the world leper colonies, leprosaria, sanatoria or asylums were created to isolate sufferers. By 1225 there were around 19,000 in Europe. In medieval England, at least 345 hospitals were wholly or in part for lepers. In 1816 Johan Ernst Welhaven (1775-1828) exposed the shameful living conditions at the 400-year-old St Jørgen’s Hospital in Bergen, as a “graveyard for the living.” But humane reforms were slow to materialise.
Hansen’s second major contribution was to encourage new laws, introduced in Norway in 1877 and in 1885 requiring that lepers be isolated in hospitals to stop them from spreading the disease. This proved effective and was confirmed in 1897 at the First International Leprosy Congress.
The first effective treatment (the sulphone, promin) became available in the 1940s. In 1945, Robert Greenhill Cochrane (1899-1985) a British leprologist studied sulphonamide derivatives, and was among the first to use dapsone (diaminodiphenyl sulfone) c.1947-9 in leprosy [19,20].
The search for effective drugs yielded clofazimine and rifampicin in the 1960s and 1970s [21]. Shantaram Yawalkar and his colleagues formulated a multi-disease treatment (MDT), which impeded dapsone-resistant forms.
When the sulphones were introduced as the first effective treatment, many sanatoria and asylums were closed and public attitudes improved. However, new cases have not yet been eliminated. At the end of 2016 the prevalence was 0.23 per 10,000 population with 214,783 new cases (2.9 per 100 000) reported globally [22].
References
- Fulton N, Anderson LF, Watson JM, et Leprosy in England and Wales 1953-2012: surveillance and challenges in low incidence countries. BMJ Open 2016;6:e010608. doi: 10.1136/ bmjopen-2015-010608. https://doi.org/10.1136/bmjopen-2015-010608
- Rodrigues LC, Lockwood DNJ. Leprosy now: epidemiology, progress, challenges, and research Lancet Infect Dis 2011;11:464-70. https://doi.org/10.1016/S1473-3099(11)70006-8
- Pasteur ‘Recherches sur la putrefaction’, Comptes Rendus de l’Académie des Sciences 1863;66:1189-94.
- Koch, R. Untersuchungen über Bakterien: V. Die Ätiologie der Milzbrand-Krankheit, begründet auf die Entwicklungsgeschichte des Bacillus anthracis. Cohns Beitrage zur Biologie der Pflanzen 1876;2, no. 2, 277-310.
- Vogelsang TM. Gerhard Henrik Armauer Hansen 1841-1912. The discoverer of the leprosy His life and his work. Int J Lepr Other Mycobact Dis. 1978;46:257-332.
- Larsen Gerhard Henrik Armauer Hansen Seen Through His Own Eyes. International Journal of Leprosy1973;41(2):208-14.
- Hansen A. Indberetning til det Norske medicinske Selskab i Christiania om en med Understøttelse af Selskabet foretagen reise for at anstille Undersøgelser angående Spedalskhedens årsager, tildels du førte sammen med forstander Hartwig. Norsk Mag Laegevidenskaben 1874;4(Suppl.9):1-88.
- Irgens The discovery of the leprosy bacillus. Tidsskr nor Laegeforen. 2002;122(7):708-9. PMID 11998735.
- Grzybowski A, Kluxen G and Półtorak Gerhard Henrik Armauer Hansen (1841-1912) – 100 years anniversary tribute. Acta Ophthalmologica 2014;92:296-300. doi:10.1111/ aos.12159.
- https://doi.org/10.1111/aos.12159
- Danielssen DC, Boeck Om Spedalskhed Bergen, Christiania 1847, translated into French as “Traité de la Spedalskhed ou Elephanthiasis des Grecs” (Paris 1848).
- Hansen On the Etiology of Leprosy. Br Foreign Medico-chirurgical Rev 1875;55:459-89.
- Neisser Zur Aetiologie del’ Lepra. Breslauer Arzl. Zeitschr 1879;200-202;214-15. Cited by: Fite GL, Wade HW. (ref 13).
- Kobro I. Gerhard Henrik Armauer Hansen. Annals of Med 1925;7:127-32.
- Fite GL, Wade HW. The contribution of Neisser to the establishment of the Hansen bacillus as the etiologic agent of leprosy and the so-called Hansen-Neisser Int J Lepr 1955;23:418-28.
- Marmor The ophthalmic trials of G. H. A. Hansen. Surv Ophthalmol 2002;3:275-87. https://doi.org/10.1016/S0039-6257(02)00285-0
- Neisser, A. Weitere Beitrage zur Aetiologie del’ Lepra. Vorliiufige . Archiv f. pathol. Anat. 1881;84:514. https://doi.org/10.1007/BF01937671. https://doi.org/10.1007/BF01937671
- Hansen GHA, Looft Leprosy: in its clinical and pathological aspects. Bristol: J. Wright; 1895.
- https://doi.org/10.1097/00000441-189511000-00008
- Hunglison The Practice of Medicine; Or, A Treatise on Special Pathology and therapeutics, Volume 2, Lea & Blanchard, 1842, Fig 4:
- Zhu YI, Stiller MJ et Dapsone and sulfones in dermatology: overview and update. Journal of the American Academy of Dermatology 2001;45(3):420-34.
- https://doi.org/10.1067/mjd.2001.114733
- Greenwood Antimicrobial Drugs: Chronicle of a Twentieth Century Medical Triumph. Oxford, OUP 2008. Pp. 196-8.
- Rees RJ, Pearson JM, Waters MF; Pearson; Waters. Experimental and Clinical Studies on Rifampicin in Treatment of Leprosy. Br Med J 1970;688(1):89-92.
- https://doi.org/10.1136/bmj.1.5688.89
- WHO Global Leprosy Programme – Epidemiology. 2017. searo.who.int/entity/global_leprosy_programme/ epidemiology/en/
* 1. A specific microorganism is always associated with a given disease. 2. The microorganism can be isolated from the diseased animal and grown in pure culture. 3. The cultured microorganism will cause disease when transferred to a healthy animal. 4. The same microorganism can be isolated from the newly infected animal.