Abstract
There is recognition of the need for rehabilitation after TBI, but less for expert diagnosis at the level of pathology and impairment during rehabilitation. To minimise disability and cost and to maximise function, rigorous diagnosis of pathology and its consequences is required. A multidisciplinary Brain Injury clinic can provide a one-stop assessment, triage and subsequent follow-along for patients in the community after moderate-severe traumatic brain injury and enables prescription of the right treatment at the right time.
Introduction
TBI is the single largest cause of death and disability in under-40’s in the UK [1]. Survivors of significant TBI face physical, cognitive, emotional and behavioural problems, with long-term consequences, including loss of independence and employment; relationship breakdown; social isolation; mental illness; addiction; homelessness; and criminality. TBI reduces life expectancy and is a risk factor for suicide. As a result, TBI costs the UK approximately £15 billion per year [2].
The importance of establishing the nature of post-acute impairments after TBI, and reducing the 40-50% mortality over 15 years post injury [3], tends to be ignored because a) the result of injury and it’s biochemical cascade is regarded as an acute irreversible, non-relapsing, fait-accompli; b) over time residual impairments become less physical, and thus outside the expertise of acute care professionals; c) biomarkers to identify pathology and impairments post-injury are not fit for purpose; and d) at present, treatment by training is usually more effective than medical treatments, although anticonvulsants, anxiolytics, antidepressants and endocrine drugs may produce significant gains post-injury.
The Brain Injury clinic at NHNN is a one stop multidisciplinary assessment leading to an individualised diagnosis and management strategy for post-acute TBI patients.
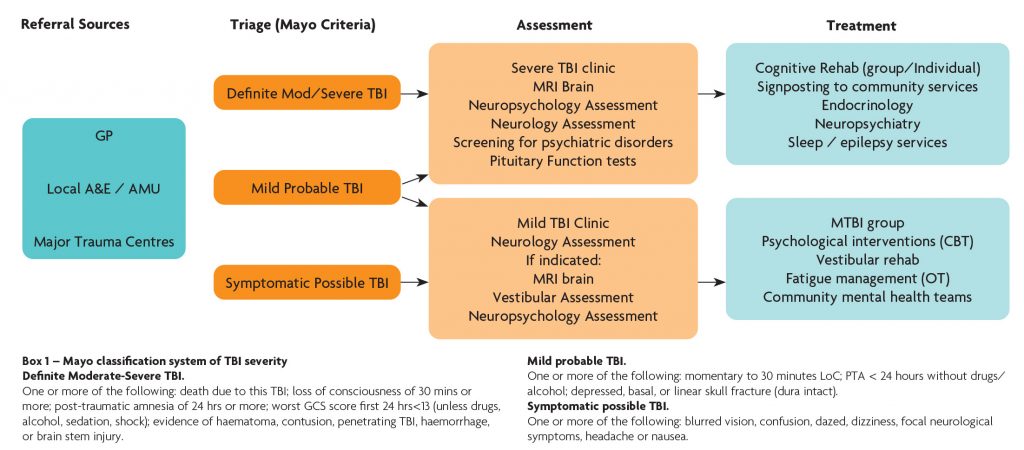
Referrals come from a variety of sources including local A&E departments and acute medical / surgical units, major trauma centres and GP’s and a proforma allowing critical information is required from all referrers. Referrals are triaged by a consultant neurologist into three categories based on the Mayo classification system of brain injury severity (Box 1). Definite moderate to severe TBI cases are triaged into the severe TBI clinic. Symptomatic possible TBI cases are triaged into the mild TBI clinic. Probable mild TBI cases are predominantly triaged to the mTBI clinic but those with prominent / severe cognitive and behavioural issues require careful investigation and are seen in the severe clinic. The clinical service reviews around 100 severe TBI and 200 mTBI cases per year.
Assessment
Comprehensive assessment generally includes neuroimaging, neuropsychological testing, neuroendocrine screening and review by a senior clinician with experience of brain injury presentations.
Neuroimaging
Almost all TBI patients have a CT of the head acutely, but many do not have an MRI. CT is sensitive to skull fractures, acute focal brain injury and intracerebral haemorrhage, and allows identification of many patients who have had a moderate/severe injury [4]. Standard MRI done post-acutely can demonstrate focal brain damage. However, normal CT or MRI can be falsely reassuring as they are insensitive to microvascular injuries which are a marker of diffuse axonal injury (DAI), an important factor producing poor clinical outcomes. More advanced MRI techniques such as susceptibility weighted imaging (SWI) show microbleeds, which are a stable marker of white matter injury after TBI [5], and should form part of the routine radiological investigation of TBI, but likely still underestimate the presence of DAI.
Neuropsychology
Neuropsychological assessment is the gold standard for investigation of cognitive, emotional and behavioural dysfunction following TBI. Standardised tests of general intellectual and focal cognitive functioning determine a patient’s profile of strengths and weaknesses relative to expected premorbid functioning. Particular attention should be paid to patterns of dysfunction associated with diffuse axonal injury and frontotemporal damage, namely attention, information processing, memory and executive functioning. Tests with higher ecological validity such as the Behavioural Assessment of the Dysexecutive Syndrome [6] give greater sensitivity to everyday executive dysfunction. Assessment of premorbid and current psychological functioning is necessary to delineate the contribution of psychological factors to cognitive test performance. Behavioural rating scales that capture both informant- and self-report, such as the Dysexecutive Questionnaire or the Frontal Systems Behaviour Scale, enable screening for a wide range of behavioural changes, including apathy, impulsivity and social disinhibition, and give a measure of self-awareness by analysing self vs. informant score discrepancies. Finally, given the range of likely co-morbid somatic, psychological and social factors that may affect test performance, assessment of response validity is required.
Neuroendocrine
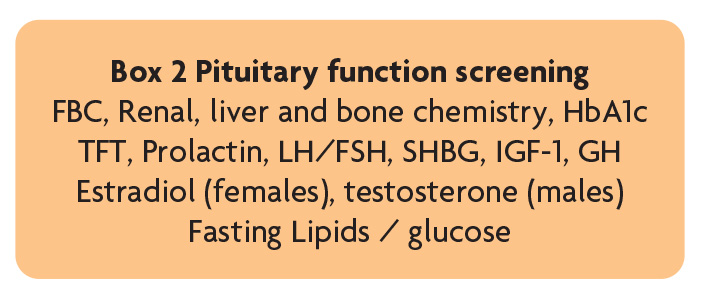
Pituitary dysfunction is a potential complication of TBI that if undiagnosed can have major physical and psychological consequences and negatively influence rehabilitation outcomes. The British Neurotrauma Group recommend those requiring admission for >48 hours following TBI (or those with symptoms of pituitary dysfunction – including fatigue, generalised weakness, low mood/motivation, reduced appetite, weight gain/loss, postural hypotension, sexual dysfunction, oligo/amenorrhea) – undergo screening at 3–6 months to exclude pituitary dysfunction (Box 2), and those with abnormal screening need endocrinology review [7] and additional dynamic testing. Our experience is that clinically relevant neuroendocrine dysfunction is relatively uncommon in this patient group but where present treatment can lead to significant improvement in outcome.
Making the correct diagnosis
A detailed history of the initial injury and immediate subsequent events is crucial to making the correct diagnosis, the injury mechanism and whether significant brain injury has occurred. Loss of consciousness for >30 minutes and/or a GCS <13/15 post injury are markers of moderate to severe TBI [4]. Duration of post traumatic amnesia (PTA) is a marker of brain injury severity, and prolonged PTA of > 2 weeks is associated with poor neurocognitive outcome and functional recovery [8]. The failure to routinely estimate PTA prospectively means retrospective estimation is often required but must be used cautiously as factors other than TBI around the time of the injury, such as analgesics, anaesthesia required for surgery, and the development of acute, or post-traumatic, stress disorder, can influence recall [9].
Careful enquiry regarding common symptoms after TBI (headache, sleep disturbance, anosmia, dizziness/imbalance, syncope/seizures, visual/auditory disturbance, fatigue, psychiatric and cognitive symptoms) allows precise diagnosis of the cause of symptoms (e.g. post-traumatic migraine, vestibular disorders, epilepsy), whether they have a physiological or structural origin, and enables individualised investigation and management.
If diagnosis is incorrect, treatment will be inappropriate, and disability and the costs of treatment will fail to diminish. This is exemplified by the 3 cases below:

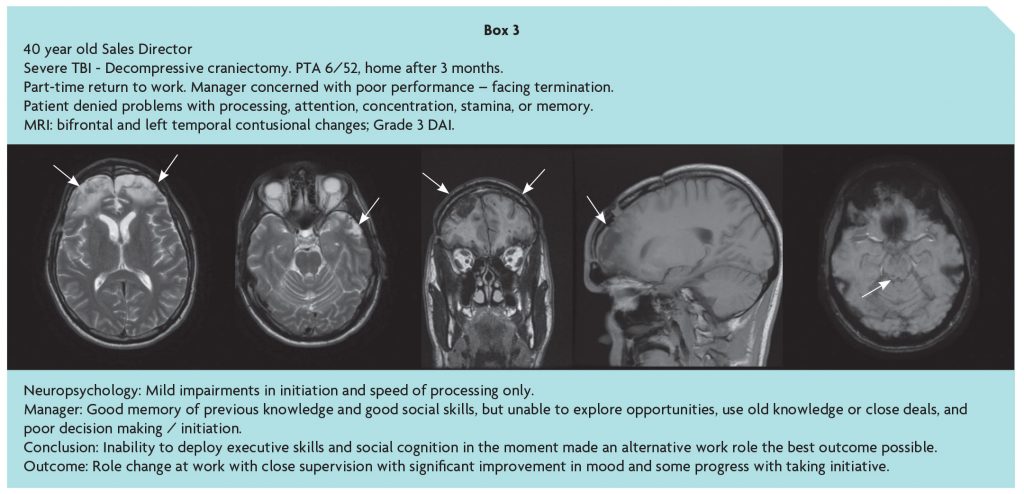
Following moderate-severe TBI, the ability to respond to the cognitive, emotional and social significance of environmental events, and return to community independence and productive activity, is confounded by disorders of social behaviour resulting from damage to frontal networks. This may escape notice in a structured inpatient setting or outpatient assessment, and in the community, especially if social skills are relatively preserved, and obscure the frontal paradox [10] and ‘hidden handicap’ of brain injury. While fronto-temporal damage may be evident on MRI, the consequences are difficult to predict, and the history given by the patient and conventional neuropsychological assessment may be misleadingly unremarkable, stressing the importance of an account of the person’s problems from a third party as illustrated in Box 3 [11].
Management
Neuropsychology
Psychoeducation is at the core of neuropsychological rehabilitation and aids understanding and acceptance of the long-term impacts of TBI. We offer a six-week group intervention incorporating education about TBI, fatigue, cognitive changes, psychological adjustment, relationship changes and vocational issues. This effectively delivers information and offers an opportunity to share experiences and develop self-awareness. Appropriate patients benefit from goal-focused rehabilitation using individualised interventions that include targeted remediation and training in compensatory and meta-cognitive strategies to optimise cognitive functioning [12]and/or psychological therapy to address changes in mood and promote positive adjustment to changes in role and identity [13]. The inclusion of family members promotes understanding and generalisation of gains to everyday life.
Drugs
Pharmaceutical treatment can be useful but must be based on precise diagnosis of the cause of presenting symptoms. Phenotyping headache correctly can lead to different management strategies (e.g. migraine prophylaxis or neuralgic pain modulation). Management of post traumatic epilepsy with Leviteracitam, which is commonly started during acute admissions, can worsen neuro-behavioural symptoms and other anti-epileptics are preferred. Dopaminergic replacement can be useful in managing abulia. Fatigue responds poorly to stimulant medication (modafinil, amantadine) and these medications are reserved for rare cases of post traumatic narcolepsy. Sleep promotion, to minimise daytime fatigue, for example using drugs to minimise catastrophizing anxiety and nocturnal rumination, may be helpful and screening for sleep disorders including circadian rhythm disruption and obstructive sleep apnoea is also important.
Neuropsychiatry
Neuropsychiatrists can be invaluable in treating mental disorders in patients with TBI. Stress and adjustment reactions are common as are anxiety and affective disorders such as depression. If the patient recalls their accident post-traumatic stress disorder may arise, is not infrequently missed and is usually undertreated. Functional cognitive disorders are also seen, particularly within the symptomatic (possible) mTBI group. These can often be distinguished by predominantly self-reported cognitive symptoms which can be at odds with functional performance on a day to day basis. Cognitive testing in this patient group is often characterised by variable performance (even on tasks testing similar functions) and effort. Non-neuropsychiatrists can sometimes be nervous about treating patients with TBI and post-traumatic epilepsy, so a one-off specialist assessment is often helpful to provide an overview and treatment plan.
Future directions
Diffusion-tensor imaging (DTI) quantifies the diffusion characteristics of water which are altered by changes in tissue microstructure, providing a sensitive marker of white matter injury. This has been validated in research but is not widely available clinically. DTI can help predict clinical outcome and the extent of diffusion changes correlates with cognitive impairment [14].
Social cognition (i.e. emotion perception, theory of mind and empathy) is emerging as an important target for rehabilitation in the TBI population, given its wide-ranging impact on interpersonal relationships, vocational success and social participation. However, assessment of social cognition is not part of routine neuropsychological testing. There is growing evidence for the effectiveness of both skill-specific and multidimensional social cognition interventions, although much remains to be known about mechanisms of change and optimal timing of treatment [15].
Cognitive stimulating drugs such as methylphenidate have efficacy in certain patients but limited effectiveness in others. Individual stratification using imaging to quantify dopaminergic deficits may be an avenue to pick out potential responders in the clinical setting [16]. Inclusion of a TBI clinic into major trauma and neuroscience centres is an efficient way to initiate and perpetuate a neuroscience–led pathway for the management of TBI in the community in the longer term [17].
References
- Maas AIR, Menon DK, Adelson PD et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurology 2017;16:987-1048. https://doi.org/10.1016/S1474-4422(17)30371-X
- Parsonage M. Centre for Mental Health Report. Traumatic Brain Injury and Offending. An economic analysis. 2016
- McMillan TM, Teasdale GM, Stewart E. Disability in young people and adults after head injury: 12-14 year follow-up of a prospective cohort. J Neurol Neurosurg Psychiatry. 2012;83:1086-91. https://doi.org/10.1136/jnnp-2012-302746
- Malec JF, Brown AW, Leibson CL, et al. The mayo classification system for traumatic brain injury severity. J Neurotrauma 2007;24:1417-24. https://doi.org/10.1089/neu.2006.0245
- Scheid R, Preul C, Gruber O, et al. Diffuse axonal injury associated with chronic traumatic brain injury: evidence from T2*-weighted gradient-echo imaging at 3T. AJNR Am J Neuroradiol 2003;24:1049-56.
- Wilson BA, Alderman N, Burgess PW, Emslie HC, Evans JJ. (1996). The Behavioural Assessment of the Dysexecutive Syndrome. Thames Valley Test Company: Flempton, Bury St Edmunds.
- Tan CL, Alavi SA, Baldeweg SE, et al. The screening and management of pituitary dysfunction following traumatic brain injury in adults: British Neurotrauma Group guidance. Journal of Neurology, Neurosurgery & Psychiatry. 2017;88:971-981. https://doi.org/10.1136/jnnp-2016-315500
- Ponsford JL, Spitz G, and McKenzie D. Using Post-Traumatic Amnesia To Predict Outcome after Traumatic Brain Injury. Journal of Neurotrauma. 2016;997-1004. https://doi.org/10.1089/neu.2015.4025
- Friedland D, Swash M. Post-traumatic amnesia and confusional state: hazards of retrospective assessment. Journal of Neurology, Neurosurgery & Psychiatry 2016;87:1068-1074. https://doi.org/10.1136/jnnp-2015-312193
- Worthington A. Decision making and mental capacity: resolving the frontal paradox. The Neuropsychologist 2019;7:31-5.
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognitive function after bilateral frontal lobe ablation: patient EVR. Neurology 1985;35:1731-41. https://doi.org/10.1212/WNL.35.12.1731
- Jolly A, Scott G, Sharp D, Hampshire A et al. Distinct patterns of structural damage underlie working memory and reasoning deficits after traumatic brain injury. Brain 2020;143:1158-1176. https://doi.org/10.1093/brain/awaa067
- Cicerone KD, Goldin Y, Ganci K et al. Evidence-based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Archives of physical medicine and rehabilitation. 2019;100(8):1515-1533. https://doi.org/10.1016/j.apmr.2019.02.011
- Waldron B, Casserly LM, O’Sullivan C. Cognitive behavioural therapy for depression and anxiety in adults with acquired brain injury. What works for whom? Neuropsychological Rehabilitation, 2013;23(1):64-101. https://doi.org/10.1080/09602011.2012.724196
- Vallat-Azouvi C, Azouvi P, Le-Bornec G, Brunet-Gouet E. Treatment of social cognition impairments in patients with traumatic brain injury: a critical review. Brain injury. 2019;33(1):87-93. https://doi.org/10.1080/02699052.2018.1531309
- Jenkins PO, De Simoni S, Bourke NJ et al. Stratifying drug treatment of cognitive impairments after traumatic brain injury using neuroimaging. Brain. 2019;142:2367-2379. https://doi.org/10.1093/brain/awz149
- Li LM et al. Management of traumatic brain injury (TBI): a clinical neuroscience-led pathway for the NHS. Clin Med (Lond). 2021;21:e198-e205. https://doi.org/10.7861/clinmed.2020-0336



