In Part I of this feature, Mr Aquilina described the presenting symptoms and signs of posterior fossa tumours in childhood and outlined the key clinico-pathological features of these tumours. In this second article, the management of these challenging cases is reviewed. This usually commences with appropriately timed investigations followed by surgery. The investigation and management of postoperative complications are also discussed.
Surgical management
Pre-operative preparation of a child with a posterior fossa tumour
High dose dexamethasone is commenced on presentation. This often leads to improvement in symptoms. However, symptomatic hydrocephalus (headache, vomiting, papilloedema and reduced level of consciousness) requires urgent surgical treatment. Traditionally, an external ventricular drain is inserted with a view to removing it after definitive tumour resection and resolution of the obstruction to CSF flow. Endoscopic third ventriculostomy is currently preferred in most centres, as it reduces the risk of infection associated with external drains and minimises the small risk of upward transtentorial herniation with large posterior fossa tumours.
A full craniospinal pre- and post-contrast MRI scan must be completed before surgery to ensure complete tumour staging before surgical contamination of the CSF by blood products.
Surgical technique
Midline posterior fossa tumours are resected via a midline suboccipital approach. The patient is positioned prone with the head elevated and flexed. The squamous occipital bone is exposed through a midline longitudinal incision from the external occipital protuberance to the level of the posterior arch of C1. C2 is not exposed; it is important to maintain the muscular and ligamentous attachments to this vertebra, as subsequent radiotherapy and surgery may result in progressive cervical instability. The C1 posterior arch is exposed but preserved.
A posterior fossa craniotomy is preferable to craniectomy. This reduces post-operative pain and allows better restoration of CSF flow around the foramen magnum post-operatively.1 The craniotomy is extended through the foramen magnum. The dura is opened in a ‘Y’ shaped fashion, remembering that in most young children an occipital sinus, descending in the midline from the torcula, may require ligation. Once the dura is reflected, the microscope is brought into the field and the arachnoid at the craniocervical junction is opened.
At this stage, tumour may be evident between the cerebellar tonsils. Ependymomas sometimes present a ‘tongue’ of tumour extending into the spinal canal; this can often be removed by gentle traction at the cisterna magna without resection of the C1 posterior arch. Fourth ventricular tumours are traditionally approached through the vermis. The longitudinal incision in the vermis should be as short as possible. Damage to the inferior vermis has been associated with an increased risk of cerebellar mutism; division of the superior vermis risks injury to the decussation of the superior cerebellar peduncles, which lies immediately deep to it.2
The telovelar approach avoids direct vermian incision. Dissection begins on one side, medial to the tonsil, between the tonsil and the uvula. This exposes the tumour superficially and the distal fourth ventricular floor deeply.3 The inferior medullary velum is stretched over a large tumour and often not identifiable. The telovelar approach allows exposure of the entire fourth ventricle up to the aqueduct and the foramina of Luschka laterally, allowing identification of the tumour boundary and gradual resection of the tumour bulk. As the aqueduct is unblocked rapid egress of CSF is often visible (Figure 1). The aqueduct should at this stage be covered by a cottonoid to prevent any blood or tumour falling into the third ventricle. Any tumour involving the floor of the fourth ventricle is reduced as much as possible to the level of the surrounding floor with the ultrasonic aspirator on minimal settings. Disruption of the floor is unforgiving and leads to severe neurological deficit.
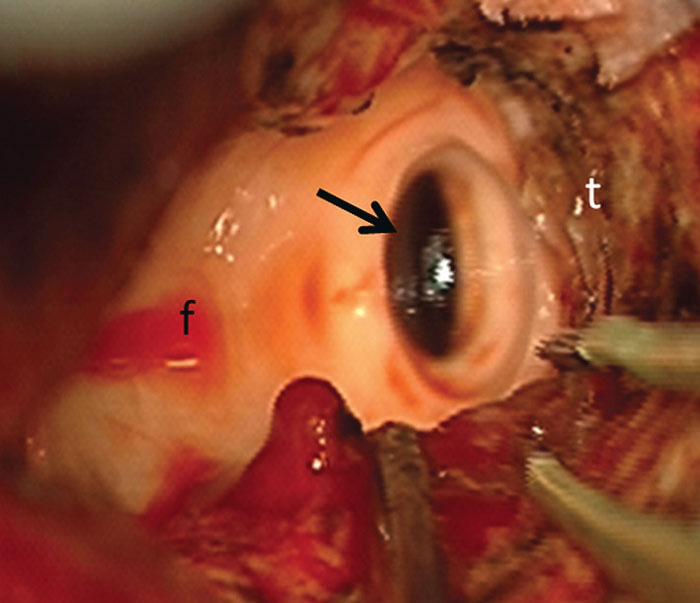
Figure 1: Intra-operative photomicrograph obtained during resection of a posterior fossa medulloblastoma –‘t’ represents residual tumour around the cavity; the fourth ventricular floor ‘f’ is exposed and free of tumour; the arrow points to the dilated caudal end of the aqueduct after decompression.
As tumour resection is completed, the walls of the cavity are carefully re-examined to ensure that no tumour has been left behind. The capsule of a pilocytic astrocytoma should also be resected if it enhances on post-contrast MRI. Haemostasis is obtained without the use of oxidised methylcellulose or other haemostatic agents; these tend to enhance on MRI and may be interpreted as residual tumour. The dura is closed in watertight fashion, usually with a patch of suturable dural substitute.
Ependymomas often extend into the cerebellopontine angle, necessitating a lateral extension to the usual midline suboccipital craniotomy. This allows use of both the fourth ventricular and retrosigmoid corridors during the primary procedure, maximising the opportunity to obtain gross total resection. These tumours infiltrate around the cranial nerves, the brainstem and the arteries of the posterior fossa. Gentle dissection, using microsuction at low setting in combination with a microdissector, proceeding in a lateral to medial direction along the cranial nerves, is necessary. These nerves, as well as perforating arteries from the basilar artery, are fragile and elongated. Patient dissection allows complete tumour resection in most cases without lower cranial nerve palsy or brainstem infarction.4
Image guidance is not usually required for midline or cerebellar posterior fossa tumours. However tumours that extend into the cerebellopontine angle or infiltrate the brainstem distort normal anatomical landmarks. Maximal safe resection is then likely to be improved with the use of neuronavigation as well as real-time per-operative imaging such as advanced ultrasound or interventional MRI.
Post-operative issues
Cerebellar mutism
Cerebellar mutism is an important complication arising after resection of midline posterior fossa tumours in children. In a review of two large clinical trials, mutism occurred in 24% of 450 children.5 Onset occurs from one to six days after surgery. A reduction in speech output, progressing to mutism, is associated with ataxia, hypotonia, irritability and emotional lability. Although it tends to improve spontaneously over two to six months, a significant proportion continues to have speech, language and cognitive deficits, as well as ataxia, one year post-operatively.5 Risk increases with medulloblastoma, brain stem invasion, and large tumours causing distortion of the brainstem, as well as in younger children.6
The precise anatomical substrate is unclear but probably involves the neuronal tracts running from the dentate nucleus through the ventro-lateral thalamus to the supplementary motor cortex.6 A recent study involving pre- and post-operative diffusion tensor imaging showed that signal abnormalities in the midbrain and superior cerebellar peduncles were more common in patients developing mutism.7 Bilateral proximal dentate-thalamo-cortical injury appeared to predispose to the condition. Changes were also evident in both fornices as well as in the white matter of the left superior frontal gyrus and right angular gyrus, suggesting a possible anatomical substrate for the behavioural abnormalities.7
Hydrocephalus
Over 80% of children with posterior fossa tumours demonstrate hydrocephalus on imaging studies at presentation.8 Postoperatively, a mean of 30% of children still have hydrocephalus, presumably related to scarring at the aqueduct or fourth ventricular outlet foramina or distortion of the fourth ventricle. These children may require endoscopic third ventriculostomy or insertion of a ventriculoperitoneal shunt. In a recent study, the risk of persistent post-operative hydrocephalus was shown to be increased in children under two, and in children with papilloedema, intracranial metastases and hydrocephalus on presentation.8
Airway and swallowing difficulties
Children with brainstem tumours or ependymomas involving the cerebellopontine angle may develop vocal cord dysfunction post-operatively, rendering them at risk of aspiration and respiratory complications. A recent study has underlined the importance of a dedicated team approach for these children, with controlled extubation on the day following surgery, once the patient is fully awake.9 The vocal cords are directly inspected by a laryngologist. In the event of bilateral vocal cord paralysis, the child is re-intubated and re-evaluated after several days. Late failure is likely to result in tracheostomy. After successful extubation, a modified barium swallow is carried out to exclude swallowing disorders.
Conclusion
As a result of clinical trials, a deeper understanding of tumour biology and progressive improvements in imaging and microsurgical techniques, the survival and outcome for children with posterior fossa tumours have improved considerably over the last 20 years. The current challenge is not just to continue to improve survival but also to reduce the impact of treatment and improve long-term quality of life, protect cognition and growth, minimise complications and reduce the risk of second malignancies in the long term.
References
- Gnanalingham KK, Lafuente J, Thompson D, Harkness W, Hayward R. Surgical procedures for posterior fossa tumors in children: does craniotomy lead to fewer complications than craniectomy? J Neurosurg 2002;97:821-6.
- Tanriover N, Ulm AJ, Rhoton AL, Jr, Yasuda A. Comparison of the transvermian and telovelar approaches to the fourth ventricle. J Neurosurg 2004;101:484-98.
- Mussi AC, Rhoton AL, Jr. Telovelar approach to the fourth ventricle: microsurgical anatomy. J Neurosurg 2000;92:812-23.
- Sanford RA, Merchant TE, Zwienenberg-Lee M, Kun LE, Boop FA. Advances in surgical techniques for resection of childhood cerebellopontine angle ependymomas are key to survival. Childs Nerv Syst 2009;25:1229-40.
- Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children’s Oncology Group. J Neurosurg 2006;105:444-51.
- Gudrunardottir T, Sehested A, Juhler M, Schmiegelow K. Cerebellar mutism: review of the literature. Childs Nerv Syst 2011;27:355-63.
- Morris EB, Phillips NS, Laningham FH, Patay Z, Gajjar A, Wallace D, et al. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain 2009;132:3087-95.
- Riva-Cambrin J, Detsky AS, Lamberti-Pasculli M, Sargent MA, Armstrong D, Moineddin R, et al. Predicting postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr 2009;3:378-85.
- Thompson JW, Newman L, Boop FA, Sanford RA. Management of postoperative swallowing dysfunction after ependymoma surgery. Childs Nerv Syst 2009;25:1249-52.
