Abstract
High grade gliomas are the most common type of brain tumours, accounting for up to 85% of all new cases of malignant primary brain tumours diagnosed every year. They carry a very large disease burden with greatest years of life lost for any cancer. Despite recent advances in the understanding of pathogenesis and management, the median survival still ranges between 12 to 18 months. Surgery along with adjuvant chemo and radiotherapy remains the mainstay of management. This review summarises the diagnosis and management as well as recent advances in the understanding and treatment of high grade gliomas.
Introduction
The term high grade glioma(HGG), is usually used to describe WHO grade III and IV tumours. The incidence of these tumours is approximately 5/100,000 person years in Europe and North America.1 Of these, glioblastomas account for 60 to 70%, anaplastic astrocytomas for 10 to 15%, anaplastic oligodendrogliomas and anaplastic oligoastrocytomas for about 10%, while anaplastic ependymoma and anaplastic ganglioglioma make up the rest.
There has been a recent increase in incidence of HGGs in the Western world, particularly in the elderly population.This probably reflects the easy availability of vastly improved diagnostic imaging.2 The median age of onset is 45 for Grade III and 60 for Grade IV tumours.3 There is a 40% greater incidence among males.4
In the majority of cases,no causative factors can be attributed. However, exposure to ionising radiation, such as from treatment of previous cancer is an established risk factor.5 Many other factors have been studied including head injury, foods with N-nitrose compounds, electromagnetic fields, smoking and most recently usage of cellular phones, all of which have not been shown to be risk factors in the development of HGG.6-8
Approximately 5% of patients with HGG have a family history of gliomas.9 Some of these are familial and associated with genetic syndromes such as neurofibromatosis types 1 & 2, LiFraumeni syndrome and Turcot’s syndrome. Recent studies have also shown links between DNA repair genes and tumour aggressiveness.10-12
Presentation
Most patients with HGG present with signs and symptoms of raised ICP from mass effect, oedema or haemorrhage. These frequently manifest as headaches, nausea & vomiting, reduced visual acuity, diplopia, drowsiness and confusion.13
Other presentations include focal neurological deficits, symptoms which are dependent on the location of the tumour such as aphasia, limb weakness, altered sensorium and neurocognitive defects including disinhibition and personality changes.14 Approximately 50% of grade III and 25% of Grade IV tumours present with seizures compared to 80% of low grade gliomas.
Imaging
The initial imaging modality is usually a contrast enhanced Computed Tomography (CT). However, Magnetic Resonance Imaging (MRI) is more sensitive than CT and is now the imaging modality of choice for all patients with brain tumours – especially if considered for surgery.
HGG typically appear as irregular, ill-defined masses with associated vasogenic oedema on both CT and MR. Enhancement typically is around the rim of the mass with a central area of non-enhancing tissue. Enhancement per se, cannot be taken as a diagnostic feature as between 30-50% of anaplastic tumours fail to enhance on CT,15 and 16% fail to enhance on MRI.16 In addition,20% of low grade gliomas (typically oligodendrogliomas) enhance on CT15 and 35% enhance on MRI16 (see Figure 1).
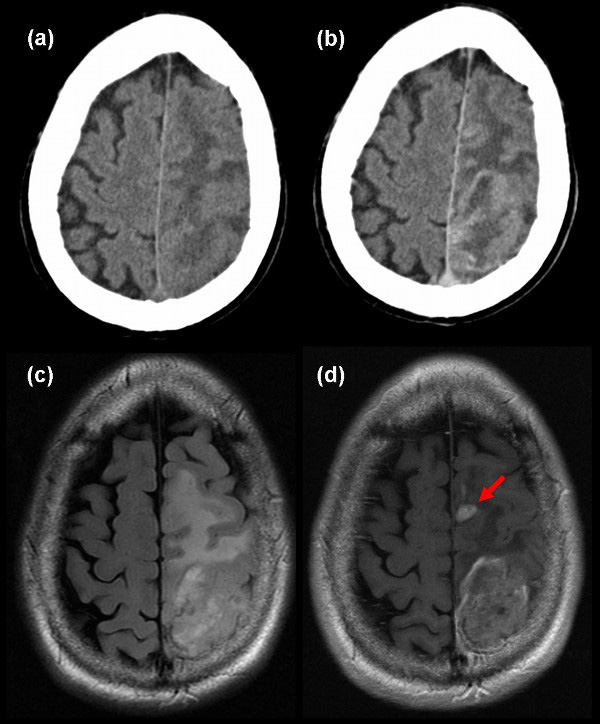
Identification of tumour margins is not possible with conventional imaging. Both post mortem and biopsy studies have shown tumour extends beyond the margin on CT,17-20 contrast-enhanced T1-weighted MRI20-21 and T2- weighted MR.20-23 These techniques cannot determine the margin or the invasiveness of these tumours. Advanced imaging methods have been developed for assessment of these tumours. These are being used in clinical practice and are summarised elsewhere.24
Post-operative MRI is the only method of objectively assessing the extent of resection. Studies have shown it identifies more cases of incomplete resection compared to the judgement of the surgeon.25 Imaging within 72 hours avoids the post-operative changes that occur later.
Histology
High grade gliomas arise from supporting glial cells in the brain. The predominant cell type determines the pathological classification. Tumours are graded according to the World Health Organisation (WHO) grading system (Grade I to IV).26 HGGs comprise of WHO grade III and IV tumours. Multiple subtypes have been identified which may alter response to treatment and prognosis. These include subtype III which comprises anaplastic astrocytoma, oligoastrocytoma, anaplastic oligoastrocytoma as well as subtype IV which includes glioblastoma, glioblastoma with oligodendrocyte component and gliosarcoma.27
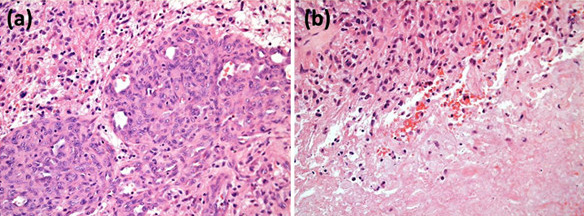
Grade III tumours are diffusely infiltrating astrocytomas with focal or dispersed anaplasia and a marked proliferative potential with increased cellularity, distinct nuclear atypia and high mitotic activity while Grade IV tumours show cellular polymorphism, nuclear atypia, brisk mitotic activity, vascular thrombosis, microvascular proliferation and necrosis (see Figure 2).
Molecular markers and their significance
Grading is determined by the most malignant part of tumour, which makes it essential for adequate sampling to determine the proper grade, especially in biopsy procedures.
One of the biggest advances in high grade glioma management has come in the form of molecular markers. Three particular markers have been well studied over the past few years.
1. MGMT Methylation Status
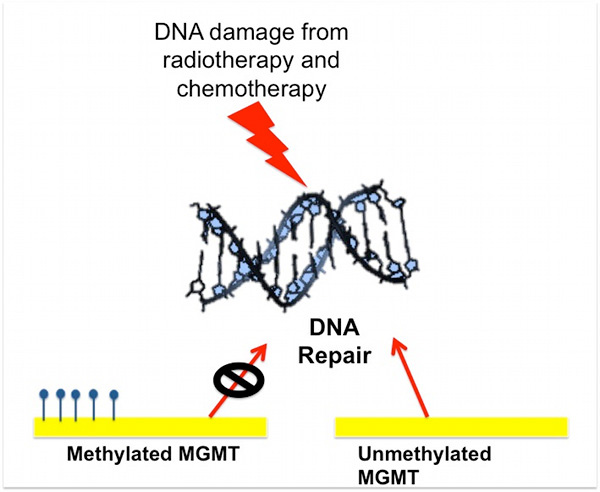
The MGMT gene encodes O-6-methylguanine- DNA methyltransferase enzyme – a DNA repair enzyme that removes alkyl groups from the O- 6 position of guanine,an important site of DNA alkylation. MGMT methylation status has been used to prognosticate response to alkylating chemotherapeutic agents like Temozolomide. In the methylated form, it is non-functional (i.e. the enzyme is not produced) thus limiting DNA repair, cf. Figure 3).
Two recent retrospective studies by Hegi et al28 and Stupp et al29 analysed this particular molecular marker and found the methylation phenomenon present in up to 45% of cases of GBM. Further, in the presence of methylation, median survival for radiotherapy with Temozolomide was significantly longer than radiotherapy alone (21.7 months, 95% CI 17.4-30.4 vs 15.3 months, 95% CI 13.0-20.9; P=0.007). In comparison, the difference in survival in the unmethylated group was insignificant (11.8 months, 95% CI 9.7-14.1 vs. 12.7 months, 95% CI 11.6-14.4). As the methylated group with radiotherapy alone has better survival than either unmethylated groups, it suggests MGMT methylation functions as a prognostic marker rather than a predictive marker. A prospective analysis by Weller et al shows an improvement in both progression free survival (7.5 months vs 6.3 months) and overall survival (18.9 months vs 11.1 months).30 MGMT methylation status assessment is not without its problems – multiple sites for mutation have been identified making this marker difficult to assess. In addition, the results are semi-quantitative with no clear cut off to define positive or negative status, resulting in non-standard interpretation of test results.
2. IDH-1 Mutation
The IDH-1 gene encodes the enzyme isocitrate dehydrogenase (IDH-1) gene that converts isocitrate to α-ketoglutarate within the Krebs cycle. This stage generates NADPH that appears to be important in dealing with cytotoxic oxidative stresses. Mutation of this gene results in an alternative metabolism of isocitrate to 2-hydroxyglutarate. Mutations cause a 10 fold increase in the production of 2-hydroxyglutarate.31 Accumulation of 2-hydroxyglutarate also leads to the breakdown of HIF-1α, a factor important for generating a malignant cell phenotype.
Mutation of IDH-1 gene was found in 12% of GBM patients and seems to confer a better median survival, (3.7 years vs 1.1years).32 Weller et al reconfirmed this via the German Glioma Network study which found IDH-1 mutations in 16/286 patients (5.6%). They also found an improvement in both progression free survival (16.2 months vs 6.5 months) and overall survival (30.2 months vs 11.2 months).30 As with the MGMT mutation, IDH-1 mutation is prognostic rather than predictive.33 Virtually all mutations (96%) are a point mutation involving arginine 132. This allows an immunohistochemistry test on paraffin embedded tissue.
3. Chromosome 1p19q Loss in Oligodendroglial Tumours
One of the commonest chromosomal abnormalities seen in oligodendrogliomas is the concurrent loss of part or all of the short arm of chromosome 1 (1p) and the long arm of chromosome 19 (19q). Cairncross et al showed loss of 1p19q in 50-70% of anaplastic oligodendrogliomas.34 This mutation had an improved survival with chemotherapy and was originally thought to be predictive of chemosensitivity. Results from a trial of radiotherapy vs chemoradiotherapy showed the radiotherapy only arm with 1p19q loss did better than the chemoradiotherapy arm without loss of 1p19q suggesting again the prognostic nature of these molecular markers.35
Pathogenesis
Glioblastomas develop either as primary tumours or arise from pre existing low grade gliomas (Table 1). These primary and secondary GBMs constitute separate and distinct disease entities. They affect different epidemiological groups and carry different prognoses.
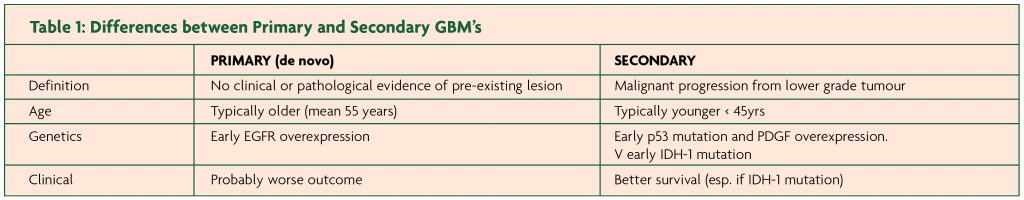
Primary gliomas usually are of a de novo manifestation involving older patients with a shorter clinical history. On the other hand, secondary gliomas are usually part of a malignant progression from low grade to high grade involving younger patients with a variable interval between 1 to 10 years.
Large numbers of genetic abnormalities have been found in gliomas.These genetic abnormalities increase as the grade of glioma increases. Most of these abnormalities affect entry into cell cycle or inhibit apoptosis via multiple pathways, which is discussed in depth elsewhere.36
Recent data from the Cancer Genome Project suggests genetic abnormalities can classify GBM’s into four categories37:
- CLASSICAL – amplification of EGFR
- MESENCHYMAL – NF1 deletion
- PRONEURAL – mutations of PDGFR-A and IDH-1
- NEURAL – expressed neuronal markers
The proneural group showed improved survival, but interestingly showed no survival advantage with chemoradiotherapy vs. radiotherapy alone. Further work is underway to understand the significance of this classification for individual patients.
Management
HGGs are best managed using a multi-disciplinary team (MDT) approach. Options for management span the spectrum from conservative treatment, to surgery and adjuvant therapy.The main thrust of management is diagnosis and prolongation of progression free survival. Dexamethasone is vitally important in controlling cerebral oedema associated with tumours. The response to steroids can be extremely rapid. Failure to improve with steroids suggests radical surgical resection may cause a worsening neurological deficit.38 The role of steroids, their complications and dosing in neuro-oncology has been recently reviewed elsewhere.39 Conservative management is a valid option in patients with a poor prognosis as identified by age and poor performance status. In this situation good palliative and supportive care is important.
Surgery
Surgery ranges from diagnostic biopsy to debulking to gross total resection.
1. Tumour Biopsy
Biopsies are minimally invasive, well tolerated and suitable for lesions of any site or size. Biopsy is usually considered when the risks of resection outweigh the benefit.40 It is best utilised in cases where initial diagnosis may influence subsequent management. However, it has its pitfalls in the form of sampling error especially in small or heterogeneous samples. To reduce the risk of non-diagnostic biopsies, image-guidance is routinely used to guide biopsies.
2. Tumour Resection
Debulking surgery is beneficial in reducing the tumour load and thus the side effects of raised intracranial pressure and providing a more representative histological sample. Whether it provides an improvement in survival is controversial. The Cochrane review of the literature has highlighted the lack of quality studies in this area.41 Retrospective studies have suggested that gross total resection as defined by post operative MRI findings of more than 98% tumour resection,has shown better quality of life and progression free survival.42 The median survival, according to one study, improved from 8 months for subtotal resection to 13 months after gross total resection (GTR).43 This was reconfirmed by Pichlmeier et al whose RPA analysis revealed survival benefit was greatest in patients with higher RPA scores (i.e more severe baseline disease based on age, performance status, neurology and mental status).The median survival for GTR vs incomplete resection was 17.7 vs 12.9 months for RPA IV and 13.7 vs 10.4 months for RPA V.44 The problem with GTR remains balancing maximal resection against potential neurological deficits.
5-ALA is a new surgical adjuvant used to identify glioma tissue under blue light (see Figure 4).
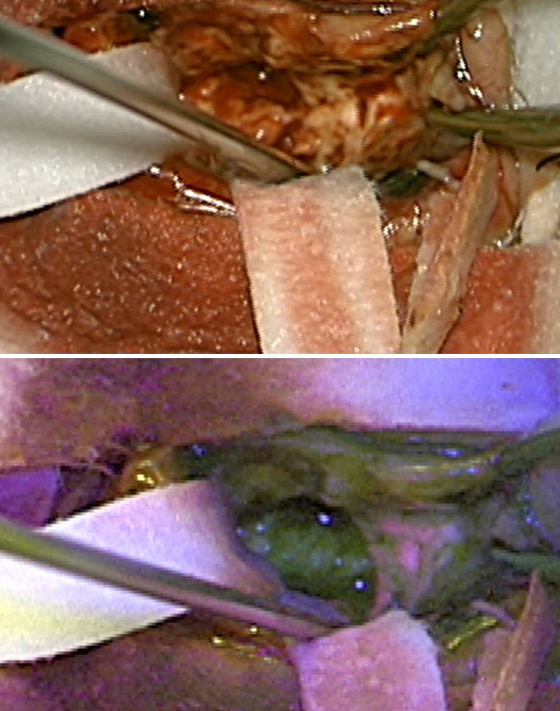
5-ALA is taken up and is converted in the normal heme biosynthesis pathway. In tumours there is a deficiency of the ferrochelatase enzyme that leads to the accumulation of the fluorophore protoporphyrin IX that fluoresces under blue light. The use of 5- ALA enables more complete resections of contrast enhancing tumour, leading to improved progression free survival in patients with malignant glioma. Stummer et al showed a 29% increase in complete resections rates in the 5-ALA group as opposed to the white light group. The 5-ALA group also had a higher 6-month progression free survival than the white light group (41% vs 21.1%).45
Another adjuvant to surgery has been local therapy with BCNU loaded wafers. These wafers provide a method of local delivery of high concentration chemotherapeutic agent bypassing the blood brain barrier. The use of these agents remain controversial though, as questions have been raised about their efficacy as well as side effects including CNS and wound infection rates as high as 28%.46,47 However, there is substantial evidence for the use of BCNU wafers in adjuvant treatment. A retrospective review on BCNU wafer implantation in combination with TMZ and radiotherapy in newly diagnosed GBM revealed a median survival of 20.7 months with a two year median survival of 36%.48
Radiotherapy
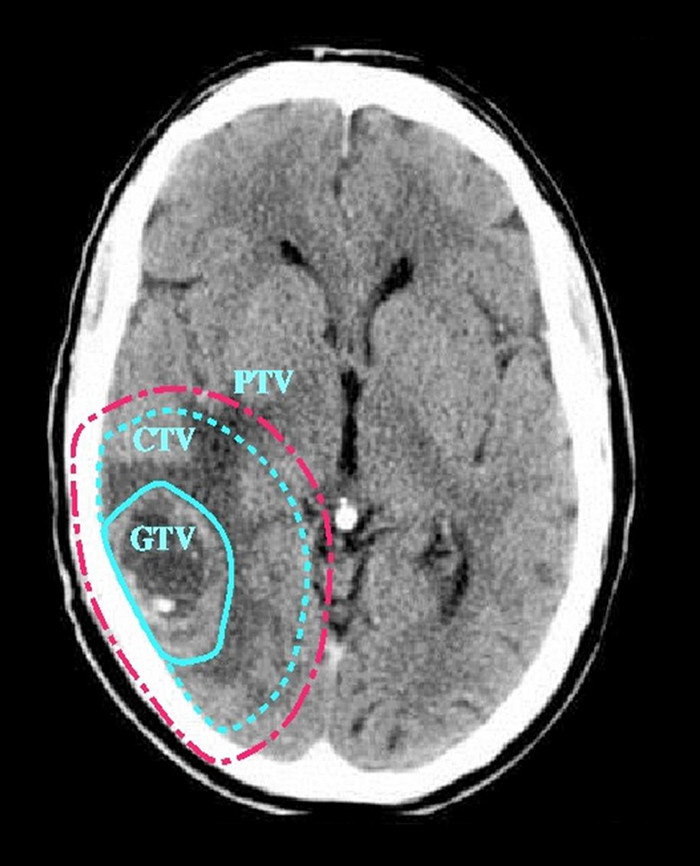
Radiotherapy has been the mainstay of adjuvant therapy for high grade gliomas since multiple studies from the 1970s showed a survival benefit.49 However, attempts to improve on the initial benefits by increasing dosage of radiation has failed to have any success. Unfortunately the therapeutic window for radiotherapy to the brain is narrow and there is an increased incidence of radiation necrosis with increased radiation dose. Part of the reason for this is the inability of conventional imaging to identify infiltrating tumour. As a result radiotherapy planning outlines the obvious tumour as the Gross Tumour Volume (GTV). A 2.5cm margin is then applied to form the Clinical Target Volume (CTV). A 0.5cm margin is added to account for set up errors and patient movement to form the Planning Target Volume (PTV). In other words, a 3cm margin is applied around the tumour that will contain normal brain.To reduce the risk of radiation necrosis the dose is therefore limited (see Figure 5).
Although various radiotherapy regimes have been proposed, traditionally two are used in HGG: 1.
1. Radical radiotherapy: gives 60 Gy dose over 30 daily fractions.
2. Short Course/Palliative Radiotherapy: gives 30 Gy over two weeks in six fractions. As there is little planning patients can start treatment very quickly and it is well tolerated. It is useful where patients are acutely deteriorating.
Cytotoxic Chemotherapy
PCV was the standard adjuvant chemotherapeutic regime in use until the advent of Temozolomide. PCV is nitrosurea based chemotherapy providing a small survival benefit; a 5% increase in two year survival rates.50 The treatment involves a 10 day oral course of procarbazine, a single oral dose of CCNU and a single intravenous infusion of vincristine given in six weekly cycles.
The introduction of Temozolomide has provided some improvement in survival. It is given orally over five consecutive days within a 28 day cycle. Leucopenia and thrombocytopenia are commonly associated side effects of this treatment. The combination of radical radiotherapy and daily, concomitant Temozolomide followed by six cycles of adjuvant Temozolomide has significantly altered the prognosis of GBM. Stupp et al. in a multi-centre trial showed that at a median follow up of 28 months, the median survival was 14.6 months in radiotherapy plus TMZ group as compared to 12.1 months with radiotherapy alone.The two year survival rate was 26.5% vs 10.4% while a five year review of the same population has revealed 9.8% survival at five years in the TMZ group compared to 1.9% in the radiotherapy group.29 This represents a significant advance over previous survival statistics in high grade glioma. The MGMT status was found to be the single most important predictive factor for a favourable outcome.
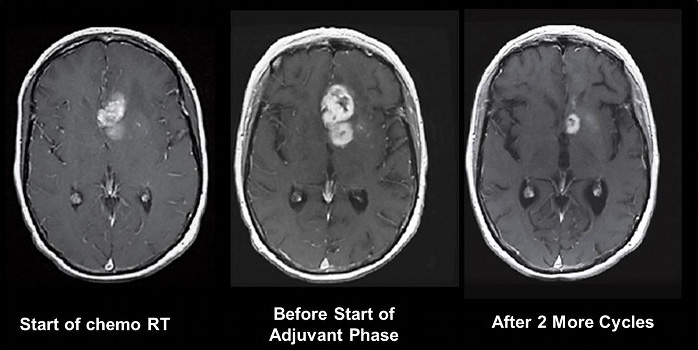
It is now well recognised that MR appearances immediately after chemoradiotherapy can show apparent progression of disease – 22% of patients in the study reported by Stupp et al did not receive the adjuvant chemotherapy phase due to presumed tumour progression. Subsequent imaging shows that these changes either stabilise or improve. This pseudoprogression is more commonly seen in patients with methylated MGMT and is thought to represent a good prognostic sign of response to therapy (see Figure 6).
Treatment at Tumour Progression
Despite the best management, virtually all patients with HGG will develop recurrence of tumour at some point. Salvage therapies may be considered depending on the patient’s clinical condition. These may include:
1. Surgery: Re-operation is associated with higher morbidity and mortality than for the original operation. However, it is an option in patients with a long progression free survival who have tumour that is maximally resectable. Insertion of carmustine wafers at this stage has been shown to improve survival.51
2. Radiotherapy: There has been some suggestion that the toxicity of re-irradiation has been overestimated. Re-irradiation is an increasingly used option with precise fractionated radiotherapy being the optimal technique. There has also been some suggestion that for accurate, focal radiotherapy to a recurrent tumour, radiosurgery may be considered. On average, time to secondary progression is in the range of several months.
3. Cytotoxic Chemotherapy: Conventional chemotherapy regimens also improve time to secondary progression; however the efficacy is only modest and treatment related toxicities like myelo-suppression occur very frequently.52 Recent Phase III Trials have failed to show that Temozolomide has a survival advantage over PCV regimes.
Prognosis
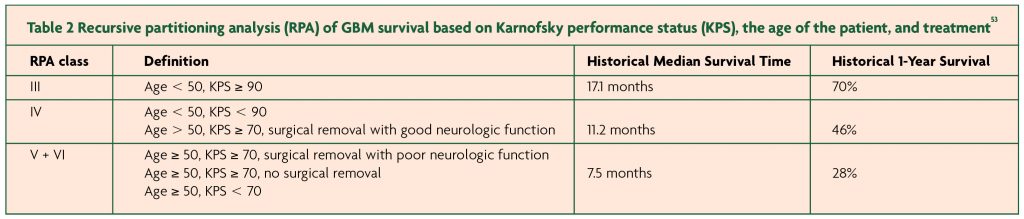
Despite advances in management, a diagnosis of HGG still carries a dismal prognosis. The median survival without any treatment is less than six months, but with treatment, this increases up to 18 months. Increasing age, poor initial neurology, poor general condition as evidenced by Karnofsky Performance score (KPS) and absence of MGMT methylation have all been associated with poor survival.53 [Table 2: RPA of GBM survival based on KPS, the age of the patient, and treatment]
Patients who undergo surgery and chemoradiotherapy have shown better long term benefits. Why certain groups of patients survive longer than others is still unknown. Death is usually due to cerebral oedema and raised intracranial pressure.
The future
Targeted molecular therapies are an exciting prospect currently at various stages of research and development. These are drugs that target the oncogenic pathways in gliomas either by interacting with receptors or affecting a downstream target. Clinical trials of drugs blocking the epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), phosphatidylinositol 3-kinase (PI3K) and related pathways and the SRC related pathways have so far been disappointing. It is clear that monotherapy will have little effect and trials combining treatments are now underway as summarised by Wick et al.54 The reason for this poor response is thought to be the fact that multiple receptor tyrosine kinases are activated in the development of a glioma,so blocking one receptor has little effect on the overall pathway.55 Combinations of targeted therapies are likely to be the way forward.
One targeted therapy of particular interest blocks the vascular endothelial growth factor pathway involved in tumour angiogenesis. A large non-randomised phase 2 study of Bevacizumab at recurrence showed a high response rate (progression free survival at six months 46%; overall survival at six months 77%).56 This trial paved the way for FDA approval. But the non-randomised nature of the study and non-standard endpoints has made the European Medicines Agency (EMEA) reject its use in Europe. One of the problems with studies using anti-angiogenic agents is the marked decrease in enhancement due to closure of the blood-brain barrier. This so called pseudoresponse does not predict the response to these agents, and subsequent progression can occur with no apparent contrast enhancement.57
References
- Central Brain Tumour Registry of the United States: Statistical report: Primary brain tumours in the United States, 2000-2004. http://www.cbtrus.org/reports/2007-2008/2007report.pdf
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;359:492-507.
- Laws ER, Parney IF, Huang W et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg 2003;99:467-73.
- National Cancer Institute, US Department of Health and Human Services, National Institutes of Health.
- Walter AW, Hancock ML, Pui CH, et al. Secondary brain tumours in children treated for acute lymphoblastic leukemia at St Jude Children’s Research Hospital. J Clin Oncol 1998;16:3761-7.
- Kleinerman RA, Linet MS, Hatch EE et al. Self-reported electrical appliance use and risk of adult brain tumours. Am J Epidemiol 2005;161(2):136-46.
- Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, DS Michaud DS. Prospective study of cigarette smoking and adult glioma: Dosage, duration and latency. Neuro-oncol 2007;9(3):326-34.
- Inskip PD, Tarone RE, Hatch EE et al. Cellular-Telephone Use and Brain Tumours. N Engl J Med 2001;344(2):79-86.
- Bondy M, Wiencke J, Wrensch M, Kyritsis AP. Genetics of primary brain tumours: a review. J Neurooncol 1994;18:69-81.
- Simon M, Ludwig M, Fimmers R, Mahlberg R, Muller-Erkwoh A, Koster G, Schramm J. Variant of the CHEK2 gene as a prognostic marker in glioblastoma multiforme. Neurosurgery 2006;59(5):1078-85.
- Bhowmick DA, Zhuang Z, Wait SD, Weil RJ. A functional polymorphism in the EFG gene is found with increased frequency in glioblastoma multiforme patients and is associated with more aggressive disease. Cancer Res 2004;64:1220-3.
- Oku MF, Selvan M, Wang LE et al. Glutathione S-transferase polymorphisms and survival in primary malignancy glioma. Clin Cancer Res 2004;10:2618-25.
- Kaye AH and Laws ER, (2001). Brain Tumours 2nd Ed Chuchill Livingstone
- Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumours. Neurosurgery 2000;47(2):324-34.
- Chamberlain MC, Murovic JA, Levin VA. Absence of contrast enhancement on CT brain scans of patients with supratentorial malignant gliomas. Neurology 1988;38(9):1371-4.
- Al Okaili RN, Krejza J, Woo JH et al. Intraaxial Brain Masses: MR Imaging-based Diagnostic Strategy–Initial Experience. Radiology 2007;243(2):539-50.
- Lilja A, Bergstrom K, Spannare B, Olsson Y. Reliability of computed tomography in assessing histopathological features of malignant supratentorial gliomas. J Comput Assist Tomogr 1981;5(5):625-36.
- Selker RG, Mendelow H, Walker M, Sheptak PE, Phillips JG. Pathological correlation of CT ring in recurrent, previously treated gliomas. Surg Neurol 1982;17(4):251-4.
- Burger PC, Dubois PJ, Schold SC, Jr. et al. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg 1983;58(2):159-69.
- Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 1987;66(6):865-74.
- Lunsford LD, Martinez AJ, Latchaw RE. Magnetic resonance imaging does not define tumour boundaries. Acta Radiol Suppl 1986;369:154-6.
- Johnson PC, Hunt SJ, Drayer BP. Human cerebral gliomas: correlation of postmortem MR imaging and neuropathologic findings. Radiology 1989;170(1):211-17.
- Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 1992;34(6):463-69.
- Price SJ. The role of advanced MR imaging in understanding brain tumour pathology. Br J Neurosurg 2007;21(6):562-75.
- Albert FK, Forsting M, Sartor K, Adams HP, Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumour and its influence on regrowth and prognosis. Neurosurgery 1994;34(1):45-60.
- du Plessis D. Primary brain tumours. ACNR 2005;4(6):17-19.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. Lyon: IARC; 2007.
- Hegi ME, Diserens AC, Gorlia T et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N Engl J Med 2005;352(10):997-1003.
- Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med 2005;352(10):987-996.
- Weller M, Felsberg J, Hartmann C et al. Molecular Predictors of Progression-Free and Overall Survival in Patients With Newly Diagnosed Glioblastoma: A Prospective Translational Study of the German Glioma Network. J Clin Oncol 2009;27(34):5743-50.
- Dang L, White DW, Gross S et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009;462(7274):739-44.
- Parsons DW, Jones S, Zhang X et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science 2008;321(5897):1807-12.
- van den Bent MJ, Dubbink HJ, Marie Y et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumours: a report of the European Organization for Research and Treatment of Cancer Brain Tumour Group. Clin Cancer Res 2010;16(5):1597-604.
- Cairncross JG, Ueki K, Zlatescu MC et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 1998;90(19):1473-9.
- van den Bent MJ, Carpentier AF, Brandes AA et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol 2006; 24(18):2715-22.
- Collins VP. Progression as exemplified by human astrocytic tumours. Semin Cancer Biol 1999;9(4):267-76.
- Verhaak RG, Hoadley KA, Purdom E et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010;17(1):98-110.
- Stummer W, Tonn J, Mehdorn HM et al. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J Neurosurg 2011;114:613-23.
- Ryan R, Booth S, Price S. Corticosteroid-use in primary and secondary brain tumour patients: a review. Journal of Neuro-oncology 2011; Oct 5. Journal of Neuro-oncology 2012;106:449-59.
- Amundson EW, McGirt MJ, Olivi A. A contralateral, transfrontal, extraventricular approach to stereotactic brainstem biopsy procedures. Technical note. J Neurosurg 2005;102:565-70.
- Hart MG, Grant R, Metcalfe SE. Biopsy versus resection for high grade glioma. Cochrane Database of Systemic Reviews Issue 2, Art. No.: CD002034. DOI:10.1002/14651858.CD002034. 2000
- Albert FK, Forsting M, Sartor K, et al. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumour and its influence on regrowth and prognosis. Neurosurgery. 1994;34:45-60.
- McGirt MJ, Chaichana KL, Gathinji M. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 2009Jan; 110(1):156-62.
- Pichlmeier U, Bink A, Schackert G et al. Resection and survival in glioblastoma multiforme: An RTOG recursive partitioning analysis of ALA study patients. Neuro-Oncology 2008;10:1025–34.
- Stummer W, Pichlmeier, Meinel T. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentrer phase III trial. Lancet Oncol 2006;7:392-401.
- Engelhard HH. The role of interstitial BCNU chemotherapy in the treatment of malignant glioma. Surg Neurol 2000;53:458-64.
- McGovern PC, Lautenbach E, Brennan PJ et al. Risk factors for postcraniotomy surgical site infection after 1,3-bis (2-chloroethyl)-1-nitrosurea (Gliadel) wafer placement. Clin Infect Dis 2003; 36:759-65.
- McGirt MJ, Than KD, Weingart JD et al. Gliadel (BCNU) wafer plus concomitant Temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg 2009;110:583-8.
- Walker MD, Alexander E, Hunt WE et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg 1978;49:333–43.
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials.
Lancet 2002;359:1011-18. - Brem H, Piantadosi S, Burger PC et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet
1995;345(8956):1008-12. - Niyazi M, Siefert A, Schwarz SB. Therapeutic options for recurrent malignant glioma Radiotherapy and Oncology 2011;98:1-14.
- Shaw E, Seiferheld W, Scott C, Coughlin C, Leibel S, Curran W, Mehta M. Reexamining the radiation therapy oncology group (RTOG) recursive partitioning analysis (RPA) for glioblastoma multiforme (GBM) patients. International Journal of Radiation Oncology Biology Physics 2003;57(2):S135-6.
- Wick W, Weller M, Weiler M, Batchelor T, Yung AWK, Platten M. Pathway inhibition: emerging molecular targets for treating glioblastoma. Neuro-oncol 2011;13(6):566-79.
- Stommel JM, Kimmelman AC, Ying H et al. Coactivation of receptor tyrosine kinases affects the response of tumour cells to targeted therapies. Science
2007;318(5848):287-90. - Vredenburgh JJ, Desjardins A, Herndon JE et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007;25(30):4722-9.
- Norden AD, Young GS, Setayesh K et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 2008;70(10):779-87.


