Abstract
Autonomic dysfunction is an increasingly recognised complication in chronic neurological conditions such as Parkinson’s disease, and other medical conditions, including diabetes mellitus, chronic fatigue syndrome, postural tachycardia syndrome (PoTS) with and without Ehlers-Danlos syndrome, fibromyalgia and recently Long COVID. Despite laboratory-based tests to evaluate normal and abnormal autonomic function, there are no home-based tests to record neuro-cardiovascular autonomic responses to common stimuli in daily life that are dependent on normal functioning of the autonomic nervous system. We have developed an adapted blood pressure/heart rate Autonomic Profile (aAP) that can be used by an individual independently and repeatedly in a domiciliary setting to determine the physiological and symptomatic response to standing, food, and physical or mental (cognitive, emotional) activities. The aAP aids separating autonomic failure (often irreversible) from autonomic dysfunction. This helps the individual and attending healthcare professional understand the relationship between symptoms and common triggers in daily life and informs on self-management in debilitating conditions such as the postural tachycardia syndrome (PoTS) and Long COVID.
The autonomic nervous system (ANS) innervates the smooth muscle of all organs, including the heart, blood vessels and various glands. It is responsible for organ function and mediating involuntary internal homeostasis, to include control of blood pressure (BP), heart rate (HR) and thermoregulation. This is unlike the somatic nervous system, which largely is under volitional control. The ANS works along with the hormonal and immunological system to respond to external and internal stimuli and ensure internal equilibrium is maintained as much as possible to enable the stable functioning of various organ systems [1]. The central ANS centres are in the brainstem, hypothalamus and cerebrum; the peripheral ANS includes the sympathetic and parasympathetic nervous systems, influencing their actions on body functions via transmitters such as acetylcholine and noradrenaline.
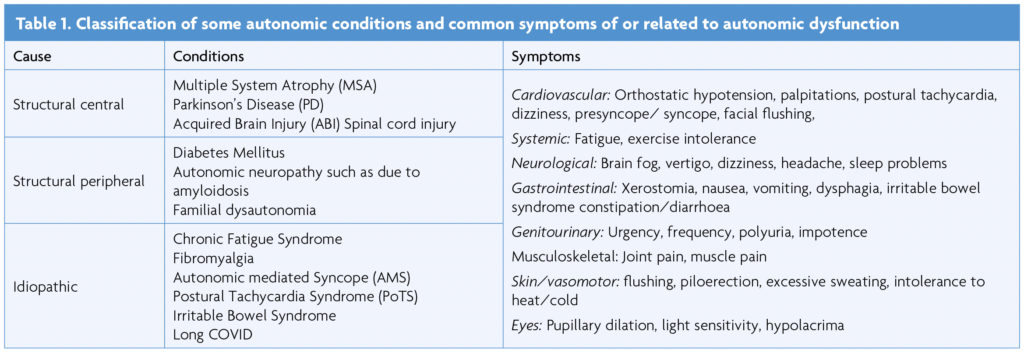
Autonomic dysfunction (AD) can result from damage to the ANS or can be idiopathic, episodic or unexplained in some cases [1] (Table 1). Long COVID is a relatively new condition and AD is reported to be present in at least a third of individuals [2,3]. There is a considerable overlap with Myalgic Encephalitis/Chronic Fatigue Syndrome (ME/CFS) where one of the features can be postural tachycardia (as in PoTS) [4]. The other common symptoms of Long COVID (breathlessness, gastrointestinal symptoms and pain) have been proposed to be linked to underlying dysautonomia, which is commonly seen in post-viral syndromes [3].
Many of the tests used for ANS evaluation are based on cardiovascular reflexes triggered by performing specific provocative manoeuvres in a controlled environment. Stimuli that alter BP or HR, such as in response to standing or passive tilting on a tilt table, and in response to the Valsalva manoeuvre, isometric exercise, cutaneous cold or heat, mental arithmetic, and deep breathing activate ANS responses that can be accurately measured [5,6]. Techniques such as measuring heart rate variability (HRV), plasma noradrenaline and adrenaline levels, sudomotor testing and microneurography test specific aspects of autonomic function [6,7].
The neuro-cardiovascular autonomic responses to postural change can be assessed in various ways. The active stand or NASA lean test measures HR and BP while standing for 10 min [8]. Measurements are made first while in the supine position. When upright, due to gravitational change, blood is redistributed to the lower extremities, which decreases venous return and cardiac stroke volume. Physiological compensatory responses through the ANS are activated to maintain adequate BP and HR. There initially is an immediate response with an abrupt fall in systolic and diastolic BP and a rise in HR (first 30s), with a phase of early stabilisation, after 1-2min, followed by a response to standing for more than 5min. The normal response includes a rise in HR by 10-15bpm and a slight decrease in systolic BP blood pressure, and in some a rise in diastolic BP by 10mmHg is normal [8,9]. A systolic BP fall of more than 20mmHg or more than 10mmHg for diastolic BP or a rise in HR of more than 30bpm (or 40/min in 12-19 yr olds) is considered abnormal [5-9].
The Head-Up Tilt (HUT) measures changes in BP and HR during and after passive head-up tilting to 60 degrees on a motorised table [10,11]. The acute fluctuations seen in the active stand-up test are not observed in this test, and it is a predominantly neuro-cardiovascular autonomic response without the influence of the pumping action of leg muscles. To ascertain the responses to food and physical exertion when upright, the head-up tilt test is combined with a relevant provocative stimulus such as a balanced liquid meal or a modified exercise test [5,7].
A variety of factors in daily life can influence neuro-cardiovascular autonomic responses, including body position, emotional state, activity (physical or mental), food ingestion, medication for specific autonomic conditions [12] and associated disorders, and other non-prescribed substances. The COVID-19 pandemic emphasised the need to develop home-based testing, as assessments of even core stimuli, such as postural change, food ingestion and physical exertion were needed with inaccessibility or considerably reduced capacity for specialised autonomic testing. The key aim was to enable the subject to conduct relevant tests themselves and report back to the clinician with their findings. This additionally enabled the individual and health care professional to understand the stimuli in daily life dependent on preserved autonomic function and evaluate factors that cause fluctuating symptoms. This is particularly relevant to Long COVID, which can be characterised by a daily and weekly fluctuation of symptoms.
Aims
To describe the adapted Autonomic Profile (aAP) test, provide a case example and discuss its role in evaluating and understanding neuro-cardiovascular autonomic dysfunction, and in aiding self-management in Long COVID and other conditions that cause autonomic impairment.
Methods
The aAP protocol was initially developed by one of the authors (CJM) in the early phase of the pandemic to test patients remotely and avoid laboratory testing and travel to hospital. It was based on experience over three decades and on information in the two autonomic departments that he developed and directed (at St Mary’s Hospital/Imperial College London and the Autonomic Unit at Queen Square, University College London). Pre-pandemic evaluation utilised ambulatory and programmed BP/HR recorders (as used in hypertension assessment), with additional measurements while lying and standing, and to food and physical exertion [13,14]. This became challenging during the pandemic as the patients needed to travel to the centre to collect the recorders and there was uncertainty about equipment sterilising procedures to avoid virus transmission. The home adapted autonomic protocol was devised to overcome these issues and was further refined by authors MS and JC for particular use in Long COVID patients. It has gone through several iterative cycles of development using feedback from physicians, therapists, researchers and especially patients who now utilise the aAP to aid diagnosis and management.
The aAP protocol
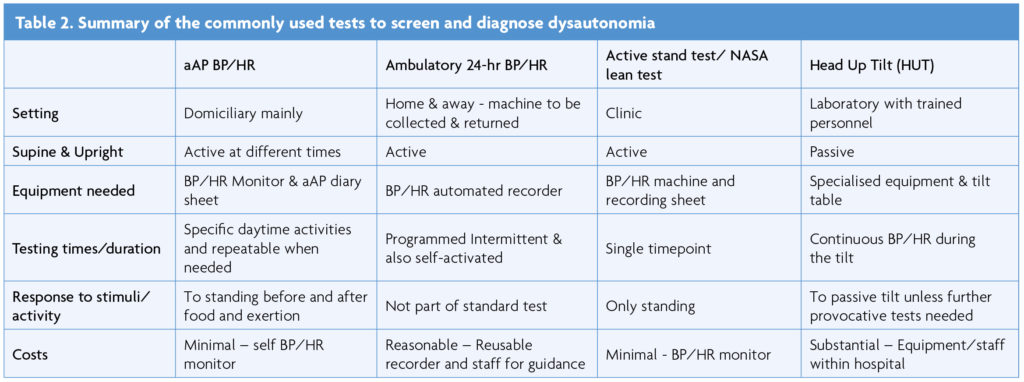
The test involves measuring BP and HR at times as outlined below while at home, with a personal arm cuff (and not wrist), BP/HR monitor as recommended by the British Heart Foundation whose website lists validated models. An example is Omron, also approved by the British Hypertension Society. The recordings provide information on neuro-cardiovascular autonomic responses to key activities in daily life such as postural change, and before and after food and exertion. Experience over the decades indicates that this protocol, even before the pandemic (from ambulatory BP/HR recorders) provided adequate data to exclude autonomic failure and dysfunction and aid initial autonomic diagnosis and guidance on treatment. The advantage with the aAP is that it can be repeated on a ‘typical’ or ‘atypical’ day and objectively can assess the response to intervention, be it non-pharmacological or pharmacological.
Unlike other standardised tests, there is no need to abstain from caffeine, nicotine, alcohol or medications for this test, as the purpose is to test in their daily life the reaction to common stimuli and record normal (or abnormal) autonomic responses to these stimuli.
To complete the aAP, time, position, BP, HR, and key symptoms in brief (such as dizziness), are recorded on the accompanying aAP diary sheet. This is of particular importance in autonomic conditions and differs substantially from BP/HR recordings commonly used for hypertension. Recordings should be taken on waking, after meals, after exertion and before sleep, with measurements taken as outlined below:
- Waking – The BP/HR is recorded after lying down, then after 3 minutes of sitting, and then after 3 minutes of standing.
- Food/liquid intake – Recordings 3-5 minutes after food ingestion at main meals (breakfast, lunch or dinner), lying down first, and then after 3 minutes of standing. A note is made of food and drink consumed (including alcohol).
- Activity – BP/HR recordings 3-5 minutes after any activity (physical, cognitive or emotional) morning and afternoon, separated from meals. Physical activity will be listed and individually differ and can be 5 minutes of walking, or going up and down a flight of stairs. Emotional activity may be watching an exciting sporting match or film. Cognitive activity may be 5 minutes working out a crossword puzzle. The subject should include at least one form of physical exertion if possible. Discussion with the clinical team will help determine the form of exercise or exertion that may be most appropriate.
In those more severely incapacitated and disabled they can substitute sitting for standing, especially if after exertion or food. If the patient wishes they can add any additional activities which cause or worsen symptoms, and which can be recorded with time, event/activity and position (lying, sitting, standing).
The patient ideally needs to choose a day when they can complete all the measurements. The intent is to provide relevant autonomic information during a standard day with usual activities so that no change in schedule is needed. The aAP can be repeated on another day if needed for comparison after adjusting for the triggers and assessing the effectiveness of any interventions.
The subject will need to be still while BP and HR is recorded. As some may feel dizzy or possibly faint especially when standing, they ideally should lean against a wall or even have another person present whilst performing the test when standing. They should abandon the recording and sit or lie down if symptoms get worse.
Box 2. Case example
A 51-year-old school teacher tested positive for COVID in September 2020. Her acute symptoms resolved but after 6 months she had ongoing breathlessness, fatigue, palpitations, dizziness, and a feeling of “butterflies” in the stomach. 24-hour ambulatory ECG demonstrated sinus tachycardia with a maximal HR of 154bpm.
She returned to work in April 2021 but with workplace modifications. Soon after her return she noticed worsening of symptoms on standing including dizziness and palpitations. Her smartwatch captured significant tachycardia and bradycardia episodes 148 and 35 respectively. A 70-degree 40-minute passive head-up tilt in Jan 2022 was inconclusive with HR increasing by 27bpm and no change in BP, with no typical symptoms reported except for transient dizziness. Provocative tests (such as food/exertion) were not carried out.
She completed an aAP in Feb 2022 which did not show significant BP changes but with certain stimuli, as indicated below, HR rose substantially fulfilling the criteria for PoTS on two occasions.
On walking, HR increased by 28bpm after 3 min standing when she was dizzy.
After lunch (ham sandwich and diet coke), HR increased by 27bpm after 3 min standing with headache.
After mild physical exertion (putting away washed clothes, up and down the stairs twice), HR increased by 34bpm after 3 min of standing (after exertion) and she was symptomatic.
After dinner (slice of toast, banana, decaf tea), HR increased by 40bpm after 3 min standing and she became symptomatic feeling breathless.
The aAP in this case was useful in providing evidence of dysautonomia through postural HR changes obtained in a home setting. It indicated a specific trigger for her symptoms, food ingestion, which she was not aware of. She was advised on post-prandial triggers, and this included eating smaller portions if needed more frequently and reducing refined carbohydrates by switching to low glycaemic index foods.
Discussion
The adapted BP/HR autonomic profile is of value in the assessment of neuro-cardiovascular autonomic dysfunction. It determines the response to various stimuli in daily life, such as postural change, food ingestion, physical activity and other activities (cognitive and emotive) that can affect such individuals. It determines if orthostatic hypotension, a marker of conditions causing autonomic damage and failure (Table 1) is present, or can be excluded. It can confirm autonomic dysfunction in Long COVID and PoTS. It helps both the healthcare professional and the individual confirm which stimuli trigger autonomic dysfunction and provides a basis for rational management. Depending on the BP and HR responses this could include adjusting the amount of food intake and limiting or changing physical exertion, which are additional stimuli that can initiate or amplify autonomic dysfunction. Importantly this can be performed in a home setting independently by the subject, and repeated when needed or if there are lifestyle or medication changes, with positive and practical interaction with the healthcare professional. This avoids the dependence on evaluation in a controlled environment, such as an autonomic laboratory. The latter is important at times for diagnosis but has limitations in the number who can be assessed and its relevance to the individual in daily life.
The aAP thresholds to confirm orthostatic hypotension and postural tachycardia (as in PoTS) remain as previously established, with the former a common finding in autonomic damage/failure and the latter in conditions where there may be no apparent structural damage, such as in Long COVID and PoTS. In Long COVID it appears commonly and with fluctuant symptoms, which often are related to specific stimuli in daily life. The aAP provides both subjective and objective evaluation of the physiological and symptom variation with common stimuli. This approach also may explain Post-Exertion Malaise (PEM) or Post-Exertional Symptom Exacerbation (PESE), both described in Long COVID which can be triggered by various activities (physical, cognitive or emotional). It may be as proposed that the immunological responses to exertion in Long COVID can worsen AD and contribute to relapse [3].
In summary, given the large number with Long COVID (> 2 million in the UK alone) and that autonomic dysfunction needs evaluating, the BP and HR aAP is a practical means of autonomic assessment and also in determining the impact of intervention in such individuals. The benefits of this test can be further evaluated in large-scale Long COVID studies such as the NIHR-funded LOCOMOTION [15].
Using the aAP protocol
The aAP self-report paper version is free to use, and the MS Word/PDF copy is available on the ACNR website or University of Leeds website.
Download the aAP Diary Sheet: PDF | Word Doc
Download the aAP Instruction Sheet: PDF | Word Doc
References
- Zalewski P, Slomko J, Zawadka-Kunikowska M. Autonomic dysfunction and chronic disease. Br Med Bull 2018;128(1):61-74. [published Online First: 2018/11/13] https://doi.org/10.1093/bmb/ldy036
- Shouman K, Vanichkachorn G, Cheshire WP, et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res 2021;31(3):385-94. [published Online First: 2021/04/17] https://doi.org/10.1007/s10286-021-00803-8
- Larsen NW, Stiles LE, Miglis MG. Preparing for the long-haul: Autonomic complications of COVID-19. Auton Neurosci 2021;235:102841. [published Online First: 2021/07/16] https://doi.org/10.1016/j.autneu.2021.102841
- Jason LA, Islam M, Conroy K, et al. COVID-19 Symptoms Over Time: Comparing Long-Haulers to ME/CFS. Fatigue 2021;9(2):59-68. [published Online First: 2021/09/07] https://doi.org/10.1080/21641846.2021.1922140
- Mathias CJ. Autonomic diseases: clinical features and laboratory evaluation. J Neurol Neurosurg Psychiatry 2003;74 Suppl 3:iii31-41. [published Online First: 2003/08/23] https://doi.org/10.1136/jnnp.74.suppl_3.iii31
- Zygmunt A, Stanczyk J. Methods of evaluation of autonomic nervous system function. Arch Med Sci 2010;6(1):11-8. [published Online First: 2010/03/01] https://doi.org/10.5114/aoms.2010.13500
- Mathias CJ, Low DA, Iodice V, et al. Investigation of autonomic disorders. In: Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. Eds. Mathias CJ, Bannister R. 5th Edition. Oxford University Press, Oxford. 2013:Chapter 22; 259-289. https://doi.org/10.1093/ med/9780198566342.003.0024
- Hyatt KH, Jacobson LB, Schneider VS. Comparison of 70 degrees tilt, LBNP, and passive standing as measures of orthostatic tolerance. Aviat Space Environ Med 1975;46(6):801-8.
- Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21(2):69-72. [published Online First: 2011/03/25] https://doi.org/10.1007/s10286-011-0119-5
- Grubb BP. Pathophysiology and differential diagnosis of neurocardiogenic syncope. Am J Cardiol 1999;84(8A):3Q-9Q. [published Online First: 1999/11/24] https://doi.org/10.1016/S0002-9149(99)00691-8
- Plash WB, Diedrich A, Biaggioni I, et al. Diagnosing postural tachycardia syndrome: comparison of tilt testing compared with standing haemodynamics. Clin Sci (Lond) 2013;124(2):109-14. [published Online First: 2012/08/31] https://doi.org/10.1042/CS20120276
- Alam M, Smith G, Bleasdale-Barr K, et al. Effects of the peptide release inhibitor, octreotide, on daytime hypotension and on nocturnal hypertension in primary autonomic failure. J Hypertens 1995;13(12 Pt 2):1664-9. [published Online First: 1995/12/01] https://doi.org/10.1097/00004872-199512010-00028
- Vichayanrat E, Low DA, Iodice V, et al. Twenty-four-hour ambulatory blood pressure and heart rate profiles in diagnosing orthostatic hypotension in Parkinson’s disease and multiple system atrophy. Eur J Neurol 2017;24(1):90-97. [published Online First: 2016/10/09] https://doi.org/10.1111/ene.13135
- Mathias CJ, Owens A, Iodice V, et al. Dysautonomia in the Ehlers-Danlos syndromes and hypermobility spectrum disorders-With a focus on the postural tachycardia syndrome. Am J Med Genet C Semin Med Genet 2021;187(4):510-19. [published Online First: 2021/11/13] https://doi.org/10.1002/ajmg.c.31951
- Sivan M, Greenhalgh T, Darbyshire JL, et al. LOng COvid Multidisciplinary consortium Optimising Treatments and servIces acrOss the NHS (LOCOMOTION): protocol for a mixed-methods study in the UK. BMJ Open 2022;12(5):e063505. [published Online First: 2022/05/18] https://doi.org/10.1101/2022.04.09.22273655


