Abstract
A new era in migraine prophylaxis has begun with the launch of antibodies blocking the Calcitonin-Gene Related Peptide (CGRP) pathway. These substances act across the entire frequency spectrum of migraine and have a tolerability superior to any other class of oral migraine preventatives based on our clinical and experimental knowledge of a 5- year period of use. Their superior tolerability profile may be due to their specificity. New questions have also arrived with these drugs ranging from the duration of therapy and treatment pause to the question of which monoclonal antibodies (mAb) for which patient – a question which we cannot answer at this stage. Nevertheless, CGRP – (R) mAbs offer a class of migraine prophylactics with significant advantages over older medications.
Migraine is a complex disorder of the central nervous system (CNS) that affects up to 15% of the adult population with a predominance in females. While the vast majority of individuals with migraine suffer from very low frequency episodic migraine (<4 monthly migraine days (MMD) /month), a significant minority that are severely affected by the disorder require pharmacological migraine prevention (<20%). These are in general individuals with chronic migraine or high frequency episodic migraine with at least 8 MMD. In subjects with low frequency episodic migraine prophylaxis can also be necessary depending on the success of single attack therapy.
The pharmaceutical treatment armentarium of prophylactics consists of oral preventatives such as topiramate, beta blockers, tricyclics and others, which were developed for other diseases and made their way into migraine by chance. Onabotulinum toxin (i.m.) is only licensed for the prevention of chronic migraine.
Drugs targeting Calcitonin gene related peptide (CGRP) or the CGRP-receptor (R) were introduced in Europe in autumn 2018. They form the only class of substances specifically designed for the prevention of migraine. All four CGRP-(R) mAbs are approved for the prevention of migraine in patients with at least 4 MMD, which includes patients with episodic and chronic migraine. Chronic migraine is defined as a headache disorder with at least 15 headache days /month of which at least 8 need to have migrainous features (for more than 3 months). Numerous analyses indicated that these drugs have significantly better tolerability than older medications [1].
Characteristics of CGRP- (R) mAbs
The first Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved CGRP antibody was erenumab, which is the only one that binds and blocks the CGRP receptor. Subsequently the antibodies blocking CGRP named galcanezumab, fremanezumab and most recently eptinezumab became available. While the first three launched antibodies are used in a s.c. formulation, eptinezumab is administered intravenously. These substances are highly specific and seem to act outside the CNS at the level of the trigeminal ganglion and/or at the node of Ranvier of primary afferent trigeminal neurons.
Possible actions may also include mast cells and meningeal arteries. Due to their molecular size, they do not penetrate the blood brain barrier in relevant amounts. CGRP-(R) mAbs are metabolised by the reticular endothelial system and are thereby devoid of liver or kidney toxicity. Typically, they do not interact with other medications. CGRP and the CGRP receptor exists in numerous tissues throughout the body.
Since CGRP and the CGRP-(R) are involved in arterial vasodilation, a potential risk of deterioration of vascular disease such as angina, myocardial infarction or stroke with the use of these mAbs was suspected prior to clinical use. These adverse events have fortunately not been described in real world studies with a clear causal relationship to the use of CGRP blocking agents. Of note one case of stroke has been described with a temporal relationship to a mAb injection [2].
A treadmill trial in males with severe cardiovascular disease, who were exposed to IV erenumab or a placebo did not show any different results between both groups, indicating that redundant mechanisms for vasodilation may exist. Whether the results of this trial in males reflect conditions in women with predominantly small vessel artery disease is a matter of debate. However, the FDA has issued a warning from post-marketing surveillance that blocking the CGRP receptor with erenumab may lead to an increased risk of hypertension or the deterioration of existing hypertension. Also, constipation has been described as a side effect of erenumab and to a smaller degree with CGRP ligand mAbs. According to clinical trials and real-world observations, constipation is typically mild and does not usually lead to treatment termination.
Other adverse events of CGRP-(R) mAbs include anaphylaxis (more pronounced when using an IV formulation), allergic reactions or local pain and swelling after injection. The worsening or development of Raynaud´s syndrome has also been described. Of note, in a mouse model of transient ischaemia two small molecule CGRP receptor antagonists led to increased infarct size due to reduced blood flow [3].
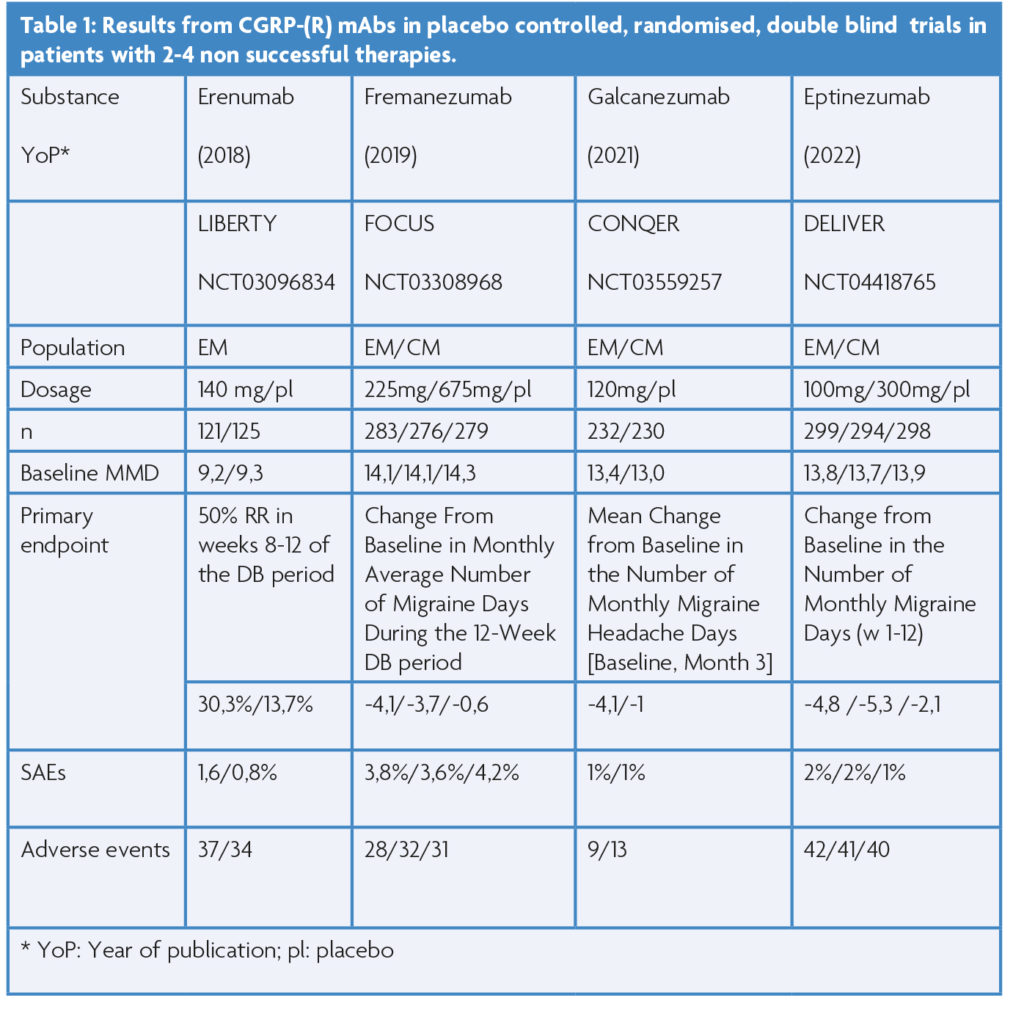
Efficacy of CGRP- (R) mAbs
In the meantime, real world studies have confirmed efficacy and tolerability data from Phase II and III placebo-controlled randomised, double-blind clinical trials (Figure 1). In fact, the efficacy of erenumab, galcanezumab and fremanezumab exceeds data from clinical trials especially in the most severely affected patient populations with frequent migraine days and numerous prior preventive unsuccessful therapies. Several groups across the globe reported convincing efficacy data in different ethnic populations.
The most severely affected patients have not been studied in placebo controlled, randomised, double-blind clinical trials. Only patients with up to four unsuccessful preventive therapies have been studied in this design (see Table 1). The first of this series of studies in episodic migraine (EM) (LIBERTY; 9.4 MMDs at baseline) with a monthly dose of erenumab s.c. 140 mg, or placebo showed that 30% of patients on erenumab reach at least a 50% reduction of MMD in the third treatment month [4].
Subsequent trials with fremanezumab and galcanezumab confirmed these findings and expanded upon the chronic migraine spectrum [5,6]. Most recently the intravenous formulation of eptinezumab completed this successful series of studies [7]. In general, the secondary endpoints were also reached in these studies. These include the reduction of acute medication use or the improvement of quality of life or total migraine burden in the CONQUER study with galcanezumab [6]. All four studies revealed that the number of prior non-successful therapies does not predict the success of CGRP- (R) mAb prophylactic therapy.
Long term follow up of the LIBERTY trial showed a consistency of efficacy up to 3 years with monthly erenumab 140mg with a 50% reduction in MMD in more than 50% of study participants. The high retention rate (>70%) of participants in the trial over three years especially indicates a beneficial tolerability and efficacy profile of erenumab. Three-year data of the CGRP antibodies in this more difficult population of individuals with migraine have not been reported. However, one-year data exist and indicate consistency of efficacy and no new adverse events.
Prior to these clinical trials, all CGRP-(R) were studied in patients with episodic and chronic migraine with up to two and three, respectively, previous preventive treatment failures, a population typically seen by headache specialists. Needless to say, all four substances passed these trials successfully. For example, the STRIVE study showed the efficacy of erenumab 70 mg and 140 mg in a six-month trial versus placebo [8]. In the galcanezumab clinical trials the loading dose of 240 mg followed by monthly doses of 120 mg were identified as the ideal treatment scheme in EM and CM for this substance based on efficacy and tolerability [9]. The EM trial by Dodick et al., showed that the efficacy of quarterly doses of fremanezumab (675 mg s.c.) is in the range of the monthly dosing scheme (225 mg s.c.) without additional side effects [10].
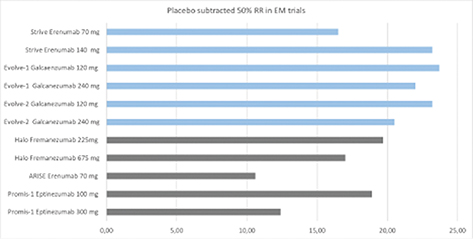
The PROMISE trials identified the 100 mg and 300mg eptinezumab IV dose as the ideal dosing scheme for this substance [11]. In all of these studies active drugs also led to a greater improvement of headache related quality of life than the placebo. It is important to note that all substances resulted in the improvement in several of the following fields: reduction of specific/unspecific acute migraine medication, work and productivity, total migraine burden and migraine related disability. All four mAbs have also demonstrated efficacy in the chronic migraine patient population [12]. Unfortunately, these trials consisted of only a three month double blind treatment phase, which is too short to explore the full efficacy of active drugs vs placebo in this patient population. However, long term open label study data in CM patients exist up to one year [13]. Most certainly the efficacy is sustained for a period of at least one year. Based on these open label trials it appears that the efficacy of CGRP-(R) mAbs increases over time in the CM population, but the lack of a placebo group does not allow us to make such statement with certainty.
In several European countries CGRP-(R) mAb therapy for migraine prophylaxis must be stopped after 9-12 months of successful therapy in order to evaluate disease modifying effects i.e. the ongoing reduction of MMDs in a drug free interval. Most recently published real world data from Italy, Switzerland and Germany showed that MMD increase from months 2 onwards after treatment termination [14]. In the 3rd month of medication pause, MMDs in most patients are close to the baseline levels before CGRP mAb initiation. In line, the quality of life deteriorates significantly during the drug holiday as assessed by HIT-6, EuroQol-5-Dimension-5-Level (ED-5D-5L) form and the Short-Form 12 (SF-12). These findings indicate that CGRP mAbs have no disease modifying effects. Raffaelli et al showed in a small sample (n=39) that 72,8% of patients responded after resumption of therapy to the same CGRP mAb they had before the medication pause [15]. Although controlled randomised studies are missing the implementation of a forced medication pause in patients successfully treated with a CGRP mAb is of limited use.
The CGRP mAbs are safe molecules in the prophylaxis of migraine with good tolerability. Recently 5-year data has been published for erenumab. In this cohort the efficacy was sustained during the entire trial period and no new adverse events were discovered [17]. In most European countries CGRP – (R) mAbs are not first line therapies for the prevention of migraine, mainly due to country specific economic reasons. However, looking at the data and comparing the CGRP- (R) mAbs to oral migraine prophylactics there is little rationale behind such a decision on scientific grounds. In fact, erenumab was studied versus topiramate in a randomised double-blind, double dummy trial in migraine prophylaxis in 777 patients with 10.4 MMDs at baseline. As expected, erenumab had a better tolerability in this trial than topiramate. However, the effectiveness of therapy was also significantly better in the erenumab group than the topiramate group [15]. Also, in study completers erenumab was more efficacious than a daily dose of 92 mg of topiramate.
In summary, CGRP mAbs offer a class of effective and tolerable migraine preventive medications, which should be considered as therapy by physicians at all times of a patient’s migraine journey.
References
- Vandervorst F, Van Deun L, Van Dycke A, Paemeleire K, Reuter U, Schoenen J, Versijpt J. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. .J Headache Pain. 2021 Oct 25;22(1):128. https://doi.org/10.1186/s10194-021-01335-2
- Aradi S, Kaiser E, Cucchiara B. Ischemic Stroke Associated With Calcitonin Gene-Related Peptide Inhibitor Therapy for Migraine: A Case Report. J Stroke Cerebrovasc Dis. 2019 Oct;28(10):104286. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.07.002
- Mulder IA, Li M, de Vries T, Qin T, Yanagisawa T, Sugimoto K, van den Bogaerdt A, Danser J, Wermer M, van den Maagdenberg A, MaassenVanDenBrink A, Ferrari M , Ayata C. Anti-migraine Calcitonin Gene-Related Peptide Receptor Antagonists Worsen Cerebral Ischemic Outcome in Mice Ann Neurol. 2020;88:771-784. https://doi.org/10.1002/ana.25831
- Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD, Klatt J. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392:2280-2287. https://doi.org/10.1016/S0140-6736(18)32534-0
- Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R, Mueller M, Ahn AH, Schwartz YC, Grozinski-Wolff M, Janka L, Ashina M. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394:1030-1040. https://doi.org/10.1016/S0140-6736(19)31946-4
- Mulleners W, Kim BY, Láinez M, Lanteri-Minet M, Pozo-Rosich P, Wang S , Tockhorn-Heidenreich A, Aurora S, Nichols R , Yunes-Medina L , Detke H. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 2020;19:814-825. https://doi.org/10.1016/S1474-4422(20)30279-9
- Ashina M, Lanteri-Minet M, Pozo-Rosich P, Ettrup A, Christoffersen CL, Josiassen MK, Phul R,Sperling B. Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): a multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2022;21:597-607. https://doi.org/10.1016/S1474-4422(22)00185-5
- Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, Sapra S, Picard H, Mikol DD, Lenz RA.A Controlled Trial of Erenumab for Episodic Migraine. N Engl J Med. 2017;377:2123-2132. https://doi.org/10.1056/NEJMoa1705848
- Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial. JAMA Neurol. 2018;75:1080-1088. https://doi.org/10.1001/jamaneurol.2018.1212
- Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, Grozinski-Wolff M, Yang R, Ma Y, Aycardi E Effect of Fremanezumab Compared With Placebo for Prevention of Episodic Migraine: A Randomized Clinical Trial. .JAMA. 2018;319:1999-2008. https://doi.org/10.1001/jama.2018.4853
- Ashina M, Saper J, Cady R, Schaeffler BA, Biondi DM, Hirman J, Pederson S, Allan B, Smith J. Eptinezumab in episodic migraine: A randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40:241-254. https://doi.org/10.1177/0333102420905132
- Tinsley A, Rothrock JF. Safety and tolerability of preventive treatment options for chronic migraine Expert Opin Drug Saf 2021;20:1523-1533. https://doi.org/10.1080/14740338.2021.1942839
- Pozo-Rosich P, Detke HC, Wang S, Doležil D, Li LQ, Aurora SK, Reuter U. Long-term treatment with galcanezumab in patients with chronic migraine: results from the open-label extension of the REGAIN study. Curr Med Res Opin. 2022;38:731-742.https://doi.org/10.1080/03007995.2022.2059975
- Raffaelli B, Terhart M, Overeem LH, Mecklenburg J, Neeb L, Steinicke M, Reuter U. Migraine evolution after the cessation of CGRP(-receptor) antibody prophylaxis: a prospective, longitudinal cohort study. Cephalalgia. 2022;42:326-334. https://doi.org/10.1177/03331024211046617
- Raffaelli B, Terhart M, Mecklenburg J, Neeb L, Overeem LH, Siebert A, Steinicke M, Reuter U Resumption of migraine preventive treatment with CGRP(-receptor) antibodies after a 3-month drug holiday: a real-world experience. .J Headache Pain. 2022;23:40. https://doi.org/10.1186/s10194-022-01417-9
- Reuter U, Ehrlich M, Gendolla A, Heinze A, Klatt J, Wen S, Hours-Zesiger P, Nickisch J, Sieder C, Hentschke C, Maier-Peuschel M Erenumab versus topiramate for the prevention of migraine – a randomised, double-blind, active-controlled phase 4 trial. Cephalalgia. 2022;42:108-118. https://doi.org/10.1177/03331024211053571
- Ashina M, Goadsby PJ, Reuter U, Silberstein S, Dodick DW, XueF , Zhang F, Paiva da Silva Lima G, Cheng S, Mikol DD. Long-term efficacy and safety of erenumab in migraine prevention: Results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol. 2021 ;2:1716-1725. https://doi.org/10.1111/ene
