Chronic subdural haematoma (CSDH) is one of the most common clinical entities encountered in daily neurosurgical practice. It generally occurs in the elderly population in whom age related reductions in brain volume with a corresponding increase in the size of the subdural space increase the vulnerability to this disease. Cerebral atrophy is also important in increasing the risk of CSDH in patients with epilepsy, alcoholism, Huntington’s disease and those with overdrainage from a ventriculo-peritoneal shunt. Patients with a coagulopathy, including antiplatelet and antithrombotic therapy (e.g. aspirin, dypyridamole, warfarin and heparin) are also at an increased risk of CSDH.
Pathogenesis (Figure 1)
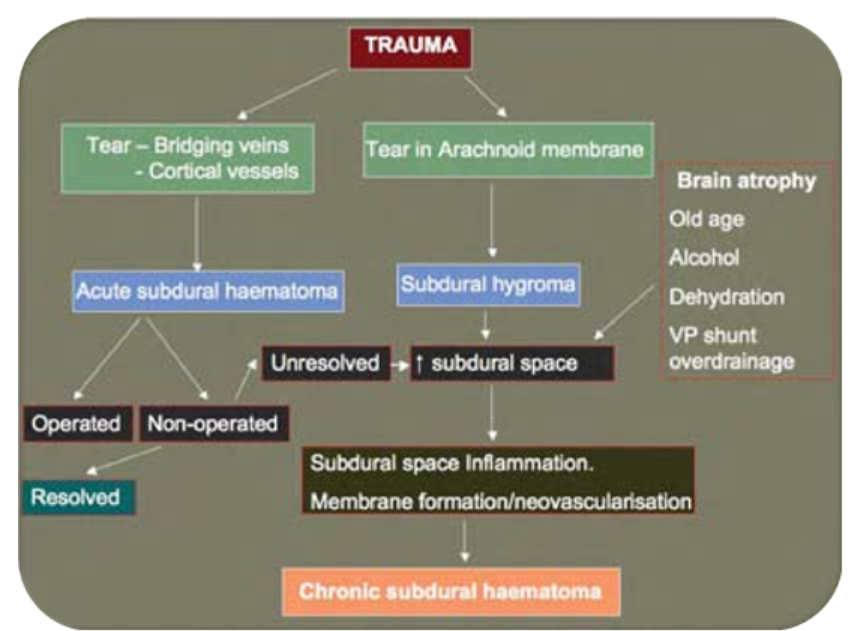
Two mechanisms, either alone or in combination, appear to play an aetiological role in the development of a CSDH.
- An acute subdural haematoma that has not been evacuated may evolve into a CSDH. As the acute haematoma matures an inflammatory membrane forms and envelopes the clot. Repeated minor haemorrhages from neovascular structures in the membrane may contribute to haematoma expansion [1]. In addition the acute haematoma liquefies within days of the initial bleed. Fluid ingress, driven by an osmotic gradient generated by fibrinolytic products within the haematoma has been postulated to cause expansion during the conversion of an acute to a chronic subdural haematoma [2,3].
- Subdural hygroma formation secondary to a traumatic tear of the cortical arachnoid membrane allowing egress of CSF into the subdural space. The further expansion of the hygroma with conversion to a CSDH is attributed to repeated minor haemorrhages into the subdural collection from the membrane that surrounds the collection [4]. The demonstration of Beta–trace protein, a highly specific CSF marker, in the subdural fluid of the vast majority of patients with CSDH and all of the patients with a subdural hygroma offers support for this hypothesis.
Presentation
- Symptoms and signs of raised intracranial pressure: headache, nausea, vomiting, impaired level of consciousness, papilloedema.
- Focal neurological deficit secondary to compression of neuronal pathways: This depends on the location of the subdural haematoma (e.g hemiplegia with a posterior frontal haematoma, dysphasia with a dominant temporal haematoma, and sensory inattention with a parietal haematoma). In clinical practice deficits, including altered level of consciousness can fluctuate in severity leading to a delay in diagnosis.
- Seizures: Focal or generalised.
Investigations
Computed tomography remains the preferred imaging modality and CSDH is classically described as a hypodense sickle-shaped extra axial fluid collection with evidence of surrounding mass effect. Where the haematoma evolves as a result of an acute bleed its density and appearance change with time in relation to the surrounding cortical surface. Three phases are described:
1. Hyperdense (0-7 days)
2. Isodense (1-3 weeks)
3. Hypodense (>3 weeks)
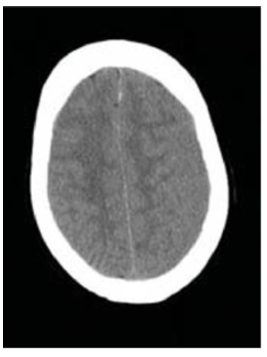
In the majority of cases a CSDH is visualised as a mass comprising hypo and hyperdense signal characteristics. Bilateral isodense SDH’s may result in a misdiagnosis due to difficulties in identifying cerebral cortex and the absence of midline shift (Figure 2). A contrast CT scan will show any enhancing membranes and can delineate the haematoma more precisely. MRI is also a useful adjunct in some cases. For the most part, T1 and T2 images both show the haematoma to be hyperintense relative to brain and CSF [6]. The change of signal intensity correlates with the length of time the haematoma has been present and the breakdown of blood in the haematoma capsule (Figure 3).
Treatment
Conservative and surgical approaches can be adopted when managing patients with a CSDH. A watch, wait and re-scan policy is usually recommended in asymptomatic or minimally symptomatic patients with a thin CSDH. Bed rest, osmotic diuretics and corticosteroids have been used although the evidence to support these measures is sparse [7]. For a patient with a symptomatic CSDH, surgery is the treatment of choice.
Preoperative workup
When undertaking surgery for a CSDH the coagulation status of the patient is of paramount importance. Aspirin should be stopped. In some cases the clinical status of the patient will necessitate urgent surgery despite aspirin treatment. If the patient’s condition is stable, neurological deficits minor and the haematoma relatively small, surgery can be delayed for a few days to permit recovery of platelet function after cessation of antiplatelet therapy.
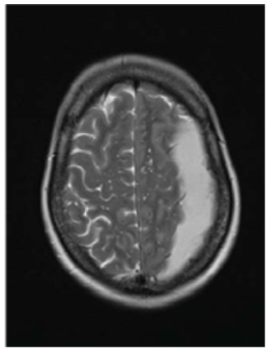
as shown on the T2 weighted sequence confirms a left sided
chronic subdural haematoma.
Another important factor in the treatment decision making process is the patient’s coagulation status especially with the widespread use of therapeutic blood thinning agents. Warfarin therapy poses specific problems. Historically, warfarin reversal comprised oral or i.v. Vitamin K administration supplemented with fresh frozen plasma. Such a process can lead to unacceptable delays in performing emergency surgery while FFP is obtained, thawed, administered and haematological parameters rechecked. Complete and rapid reversal of warfarin over- anticoagulation is better achieved with 5 or 10mg of intravenous vitamin K and II, VII, IX and X factor concentrate (Beriplex™) [8,9].
Surgical options
The surgical approaches to the management of patients with CSDH are mainly limited to burrhole drainage, twist drill drainage and craniotomy. A small craniectomy has also been advocated as an alternative approach. Combining each technique with the use of intraoperative irrigation and/or post-operative drainage provides a variety of treatment options.
Surgical Techniques
Chronic SDHs are most commonly treated by burr hole evacuation. The number and location of the burr holes depends upon the size and location of the haematoma as determined by CT scan. Two burr holes located along the same line as the incision of a trauma flap are commonly employed. Care must be taken to secure any dural bleeding. The distinctive grey encapsulating membrane is opened to permit drainage of the liquefied haematoma. This is often under considerable initial pressure. Occasional conversion to a craniotomy is required if a substantial solid component persists. Irrigation of the subdural space, facilitated by the use of a soft Jacques catheter, is commonly performed to facilitate evacuation.
Twist drill craniostomy has been advocated in search of a less invasive treatment option with a skull opening usually less than 5mm. However, irrigation through such a small aperture is difficult. A craniotomy permits fluid evacuation and partial removal of the haematoma membrane in patients with recurrent, persistent chronic subdural haematomas [10]. A small craniectomy is an alternative that enables the significance of post-operative collections to be assessed by palpation and treated by percutaneous aspiration. A valveless subdural-peritoneal conduit fashioned from a peritoneal catheter with side holes cut for the subdural space and securely anchored to the galea can be useful in the treatment of patients with an atrophic brain where persistence of the subdural collection occurs despite recent drainage. Weigel et al. analysed 48 publications (19812001) in a comprehensive meta-analysis comparing the outcome of various surgical techniques [11]. A wide range of cure, recurrence and mortality rates were found with each procedure. Overall, there was no significant difference in mortality between the three techniques. Mortality of up to 11% was noted. The morbidity from a craniotomy was reported to be higher than drainage procedures. Comparison between burrhole drainage and twist drill craniostomy revealed a higher recurrence rate with a twist drill approach.
Irrigation and drainage
The use of intraoperative irrigation with warm isotonic saline or artificial CSF until the effluents are clear is widespread. No significant differences were reported in the patients who received irrigation compared with those who did not in several series [12-15]. In a twist drill series, irrigation was found to improve the recurrence rate from 29.2% to 6.7% [16]. In contrast a lower recurrence rate has been reported in a more recent series in patients in whom intra-operative irrigation was not used [17].
The use of closed post-operative subdural space drainage has long been considered. Some prospective studies showed no beneficial effect, [10,18,19] whereas other authors report lower recurrence rates with the use of post-operative drains [20]. In patients undergoing twist drill craniostomy, post-operative drainage did appear to reduce the recurrence rate from 68% to 9% in a meta-analysis [11]. The use of a closed subgaleal drainage system has also been reported [21]. Whilst there are no reports of a proven increased risk of infection with drains, this concern must exist.
Peri-operative continuous inflow and outflow irrigation with Ringer’s lactate solution after evacuation of the haematoma has been reported to reduce the recurrence risk in a small prospective randomised study [22]. However the differences did not reach statistical significance (1/19 vs 4/18). A retrospective comparison of post operative inflow and outflow drainage with burr hole evacuation +/- closed drainage and craniotomy showed significantly lower recurrence rate in patients with continuous inflow-outflow drainage [23]. Despite these small studies such techniques have not been widely adopted.
Complications
Postoperative CT scans to follow the progress of patients have shown that residual haematoma is quite common regardless of the operative procedure used. However, in the majority of cases, removal of most of the haematoma will result in alleviation of symptoms and any residual haematoma will gradually reabsorb over a period of weeks.
The incidence of true reaccumulation or recurrence of the haematoma varies with the chosen surgical intervention as discussed above. Many risk factors for recurrence of CSDH have been reported previously, including advanced age, bleeding tendency, brain atrophy, haematoma density, alcohol abuse, postoperative subdural air accumulation, bilateral CSDH and arachnoid cyst. More recently the presence of high concentrations of beta trace protein in the subdural fluid at the time of initial surgery signifying CSF leakage into the subdural space, and high levels of interleukin-6 signifying inflammation of membranes or enhanced expression of VEGF and bFGF in the outer membrane may result in a higher risk of a recurrence [5,24].
Other complications include seizures, pneumocephalus, subdural empyaema and rarely intracranial haemorrhage. Extracranial complications such as post-operative pneumonia and pulmonary embolism may also occur in patients with a CSDH. Following surgery the risks and benefits of antiplatelet and anticoagulant therapy need careful consideration on a case by case basis before reintroduction.
Summary
The diagnosis of chronic subdural haematoma should be considered in any elderly patient presenting with focal neurological signs or with any suggestion of raised intracranial pressure. For the majority of patients surgical drainage of a symptomatic chronic subdural haematoma is readily performed with a relatively low risk of operative morbidity and mortality. Most patients make a satisfactory recovery. Reaccumulation is the most common sequelae and can be troublesome in a small minority of patients. A number of operative variations have been reported to try and minimise this risk, however the evidence to support any specific operative technique is not persuasive. The use of post-operative anticoagulants and antiplatelet agents needs careful consideration particularly in patients with a history of haematoma recurrence.
References
- Stoodley M, Weir B. Contents of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11(3):425-34. https://doi.org/10.1016/S1042-3680(18)30104-9
- Drapkin AJ. Chronic subdural hematoma: pathophysiological basis for treatment. Br J Neurosurg. 1991;5(5):467-73. https://doi.org/10.3109/02688699108998475
- Weir B, Gordon P. Factors affecting coagulation: fibrinolysis in chronic subdural fluid collections. J Neurosurg. 1983;58(2):242-5.https://doi.org/10.3171/jns.1983.58.2.0242
- Lee KS. Natural history of chronic subdural haematoma. Brain Inj. 2004;18(4):351-8. https://doi.org/10.1080/02699050310001645801
- Kristof R, Grimm J, Stoffel-Wagner B. Cerebrospinal fluid leakage into the subdural space:possible influence on the pathogenesis and recurrence frequency of chronic subdural haematoma and subdural hygroma. J Neurosurg. 2008;108(2)(Feb):275-80. https://doi.org/10.3171/JNS/2008/108/2/0275
- Hosoda K, Tamaki N, Masumura M, Matsumoto S, Maeda F. Magnetic resonance images of chronic subdural hematomas. J Neurosurg. 1987;67(5):677-83. https://doi.org/10.3171/jns.1987.67.5.0677
- Sun TF, Boet R, Poon WS. Non-surgical primary treatment of chronic subdural haematoma: Preliminary results of using dexamethasone. Br J Neurosurg. 2005;19(4):32733. https://doi.org/10.1080/02688690500305332
- Baglin TP, Keeling DM, Watson HG. Guidelines on oral anticoagulation (warfarin): third edition–2005 update. Br J Haematol. 2006;132(3):277-85. https://doi.org/10.1111/j.1365-2141.2005.05856.x
- Evans G, Luddington R, Baglin T. Beriplex P/N reverses severe warfarin-induced overanticoagulation immediately and completely in patients presenting with major bleeding. Br J Haematol. 2001;115(4):998-1001. https://doi.org/10.1046/j.1365-2141.2001.03214.x
- Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981;54(5):637-45. https://doi.org/10.3171/jns.1981.54.5.0637
- Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003;74(7):937-43. https://doi.org/10.1136/jnnp.74.7.937
- Iwadate Y, Ishige N, Hosoi Y. Single burr hole irrigation without drainage in chronic subdural hematoma. Neurol Med Chir. (Tokyo) 1989;29(2):117-21. https://doi.org/10.2176/nmc.29.117
- Benzel EC, Bridges RM, Jr., Hadden TA, Orrison WW. The single burr hole technique for the evacuation of non-acute subdural hematomas. J Trauma. 1994;36(2):190-4. https://doi.org/10.1097/00005373-199402000-00007
- Matsumoto K, Akagi K, Abekura M, Ryujin H, Ohkawa M, Iwasa N, et al. Recurrence factors for chronic subdural hematomas after burr-hole craniostomy and closed system drainage. Neurol Res 1999;21(3):277-80. https://doi.org/10.1080/01616412.1999.11740931
- Suzuki K, Sugita K, Akai T, Takahata T, Sonobe M, Takahashi S. Treatment of chronic subdural hematoma by closed-system drainage without irrigation. Surg Neurol. 1998;50(3):231-4. https://doi.org/10.1016/S0090-3019(97)00339-X
- Aoki N. Subdural tapping and irrigation for the treatment of chronic subdural hematoma in adults. Neurosurgery. 1984;14(5):545-8. https://doi.org/10.1097/00006123-198405000-00003
- Kuroki T, Katsume M, Harada N, Yamazaki T, Aoki K, Takasu N. Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir (Wien). 2001;143(10):1041-4. https://doi.org/10.1007/s007010170010
- Markwalder TM, Seiler RW. Chronic subdural hematomas: to drain or not to drain? Neurosurgery. 1985;16(2):185-8. https://doi.org/10.1227/00006123-198502000-00010
- Laumer R, Schramm J, Leykauf K. Implantation of a reservoir for recurrent subdural hematoma drainage. Neurosurgery. 1989;25(6):991-6. https://doi.org/10.1227/00006123-198912000-00026
- Wakai S, Hashimoto K, Watanabe N, Inoh S, Ochiai C, Nagai M. Efficacy of closed-system drainage in treating chronic subdural hematoma: a prospective comparative study. Neurosurgery. 1990;26(5):771-3. https://doi.org/10.1227/00006123-199005000-00006
- Gazzeri R, Galarza M, Neroni M, Canova A, Refice GM, Esposito S. Continuous subgaleal suction drainage for the treatment of chronic subdural haematoma. Acta Neurochir (Wien). 2007;149:973-4. https://doi.org/10.1007/s00701-007-1139-8
- Ram Z, Hadani M, Sahar A, Spiegelmann R. Continuous irrigation-drainage of the subdural space for the treatment of chronic subdural haematoma. A prospective clinical trial. Acta Neurochir (Wien). 1993;120(1-2):40-3. https://doi.org/10.1007/BF02001467
- Hennig R, Kloster R. Burr hole evacuation of chronic subdural haematomas followed by continuous inflow and outflow irrigation. Acta Neurochir (Wien) 1999;141(2):171-6. https://doi.org/10.1007/s007010050282
- Hong H, KIM Y, Yi Y, Ko Y, Oh S, Kim J. Role of angiogenic growth factors and inflammatory cytokine on recurrence of chronic subdural haematoma. Surg Neurol 2008;17(Apr):(Epub ahead of print).



