Abstract
Guillain-Barré Syndrome (GBS), or Acute Inflammatory Demyelinating Polyneuropathy (AIDP) is a rare, acquired condition which can cause pain, sensory impairment and weakness in the limbs. The severity varies widely from mild non-disabling symptoms to complete flaccid tetraplegia, respiratory failure and autonomic instability. Those with severe weakness are particularly susceptible to the complications of immobility, most prominently shortening of the soft tissues and potentially permanent loss of range in the joints. Here, we set out a multi-disciplinary approach to rehabilitating people with GBS , which we consider as three processes: prevention, adaptation and restoration. We describe how the approach should be tailored and used flexibly over the course of the person’s rehabilitation, aiming to maximise recovery and minimise long-term disability.
Key points
- The rehabilitation of people with Guillain-Barré Syndrome can be considered as three processes: Prevention, Adaptation and Restoration.
- A comprehensive rehabilitation programme will often require all three processes, however the proportion of each will depend on the degree of nerve regeneration and the presence of factors such as pain and fatigue.
- Early intervention is essential to maintain joint range of movement and prevent secondary complications of immobility.
- Ongoing access to community and/or outpatient services is vital to reduce long-term disability, optimise the transition from a hospital setting to the community and increase participation in leisure, work and social activities.
Introduction
Guillain-Barré syndrome (GBS), or Acute Inflammatory Demyelinating Polyneuropathy (AIDP) is a rare neurological disorder estimated to effect 1-2 people in 100,000 per year [1]. It is usually preceded by infection or other immune stimulation that triggers an autoimmune response targeting peripheral nerves and their spinal roots [2]. Demyelination of the peripheral nerves results, which in most cases may be repaired quickly by Schwann cells. However, in more severe cases there may be loss of nerve axons leading to a more prolonged clinical course. The severity of GBS can vary from mild, temporary weakness to severe and chronic paralysis; 30% of people require mechanical ventilation [3] and up to 20% are unable to walk unaided 6 months after onset [4].
The management of people with GBS can be challenging as there are several variants, classified by the pattern of peripheral nerve involvement. Current literature supports a multidisciplinary team (MDT) approach, early rehabilitation and aggressive management of potential obstacles such as soft tissue changes, fatigue, pain and psychological distress [6,7]. However, there is little data on how the MDT should implement and adjust treatment strategies in response to varied and changing impairments.
Approximately 40% of people hospitalised with GBS will require inpatient rehabilitation [8]. This group usually have a degree of axonal involvement, and hence see a slow return in motor activity from proximal to distal over many months as regeneration proceeds. This occurs at a rate of 1-3 mm per day [9]; from the anterior horn cell to an extremity may take as long as 1-2 years. Appropriately timing inpatient rehabilitation can therefore be challenging due to the slow rate and sometimes incomplete nature of nerve regeneration.
The rehabilitation of people with GBS has been previously identified in two stages [10]; an early stage to reduce the level of disability and prevent secondary complications and a later stage to improve function and participation. We propose that it is more beneficial to consider rehabilitation as a series of processes rather than stages, namely prevention, adaptation, and restoration (Figure 1).
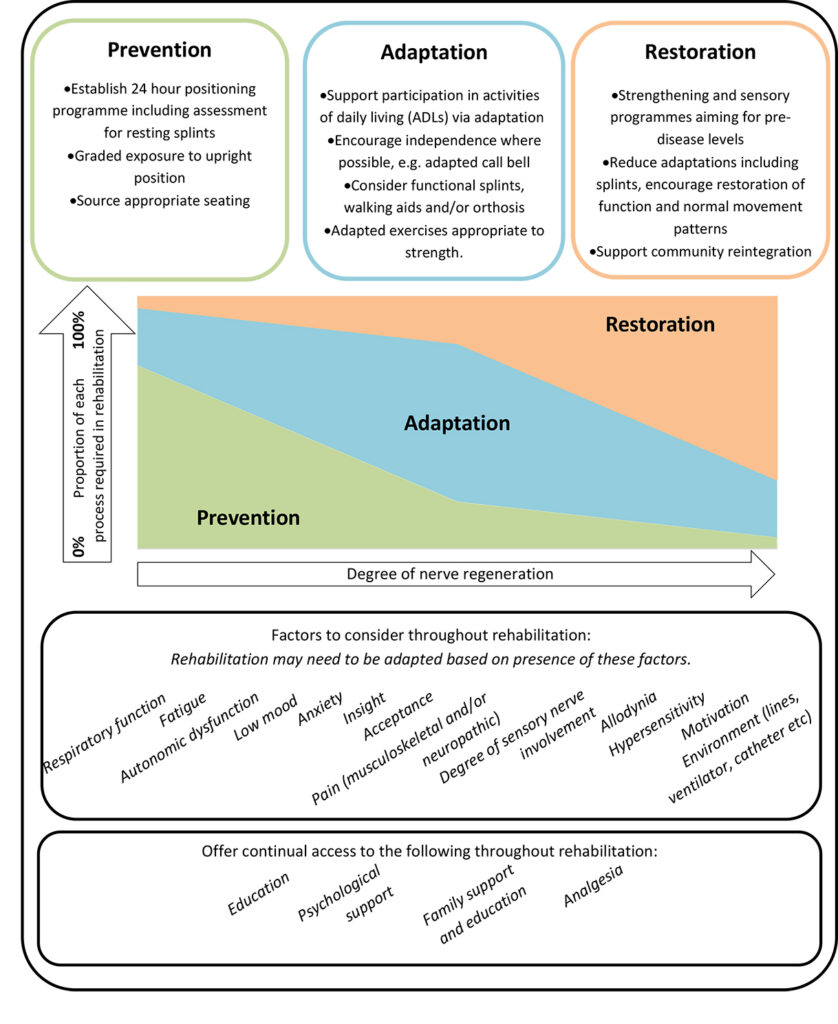
A comprehensive rehabilitation programme will often require all three processes. The proportion of each will depend on the degree of nerve regeneration and factors such as pain, fatigue, mood and psychological adjustment. For example, a person may require a positioning programme to prevent loss of finger joint range of movement but is able to brush their teeth using an adapted toothbrush. They may also have a strengthening programme aiming to restore motor power. We acknowledge that many people with GBS also suffer with respiratory complications that require management; however, this is outside of the scope of this article. This article will detail these three processes and factors to be considered throughout the course of rehabilitation.
Prevention
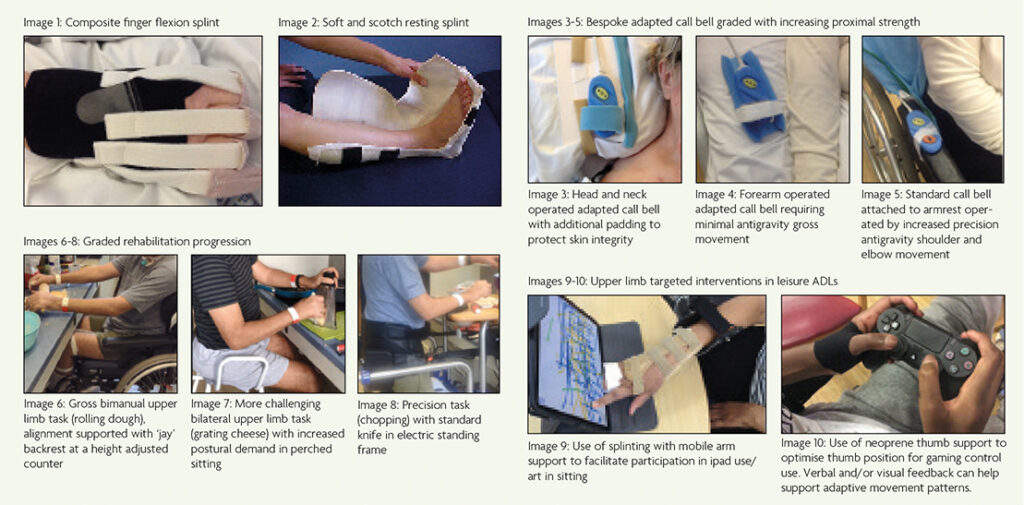
The MDT should make an early assessment of the person’s risk of developing soft tissue shortening and/or joint contracture, considering their positioning over 24-hours. Any joint that a person cannot move through a full range will be at risk, therefore those with more significant weakness will be particularly vulnerable. There is a paucity of evidence for interventions to prevent loss of joint range of movement in GBS. However, there are generally accepted management principles that may reduce this risk and associated complications including pain and pressure sores. A bespoke positioning programme should be implemented at the earliest opportunity, ensuring the resting positions of each joint are changed frequently over the 24-hour period. This may include but is not limited to use of custom and/or prefabricated splints, pillows, wedges, and other bed positioning aids (Image 1 and 2). The application of such interventions may be complicated by the environment (ventilator tubing, lines, catheter etc).
Extra care should be taken in the cases of sensory nerve involvement as the person will be more susceptible to skin breakdown and pressure sores. Allodynia and/or hypersensitivity are common and can be triggered during care tasks and repositioning. Establishing a comfortable method of handling collaboratively with the person with GBS will support desensitisation and reduce distress.
In those with facial nerve involvement, eye care is paramount to prevent secondary exposure keratosis. Ophthalmology services can provide specific recommendations however administering regular eye drops, performing manual blinks and taping the eyes closed at night will help to maintain eye health. Weakness of the facial muscles leads to muscle immobility and secondary tightness. This is a clinical area that is often overlooked yet facial massage and/or specific stretch techniques can prevent muscle tightness and reduce the longer-term impact on eating, drinking, facial expressions and psychosocial well-being.
People with GBS should gradually be exposed to upright postures, and regular periods of sitting out of bed commenced as soon as tolerated. Progression to standing can then be explored using a tilt table, standing frame or appropriate aid dependent on muscle strength. Regular periods of sitting or supported standing can then form components of the 24-hour positioning programme.
Close monitoring for signs of autonomic dysfunction is required (orthostatic hypotension, tachycardia, sweating and respiratory distress). Physical aids such as abdominal binders and compression stockings can be trialled in the first instance if orthostatic hypotension persists. If these are unsuccessful and mobilisation out of the bed cannot be tolerated, medications may be considered. These include mineralocorticoids such as fludrocortisone or sympathomimetics such as midodrine.
Adaptation
In more severe cases of GBS, people are often heavily dependent on others to perform daily tasks. Rehabilitation should focus on enabling and enhancing participation in everyday activities that are meaningful to the person. Initially, this may require an adaptive, compensatory approach.
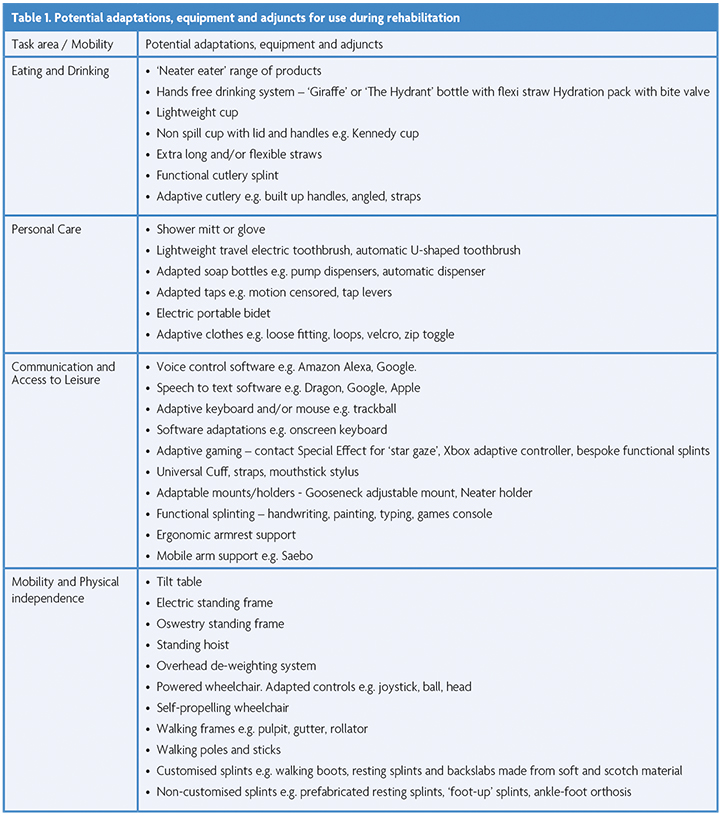
Establishing a robust means of communication at the earliest opportunity increases independence and can help to manage psychological distress, reduce isolation and alert staff to support care needs. Adapted call bells can be purchased or fabricated (Images 3-5). Voice activation, eye gaze control and facial recognition software can be used to enable independence in contacting family or friends and accessing leisure outlets such as social media, audio books and TV (Table 1). Applications can be made to ‘Guillain-Barré and Associated Inflammatory Neuropathies’ (GAIN) charity [11] for the provision of a voice-activated device. Technology support is available from organisations such as ‘AbilityNet’ [12]. Referral to local environmental control services should be considered in severe and/or prolonged cases.
Therapists should evaluate the person’s current level of functioning in daily activities to determine what limitations are present [13]. They should consider if the task or environment could be adapted to enhance participation and independence. Table 1 provides some practical examples. The ‘AskSARA’ service and ‘Living Made Easy’ website [14] and suggested product list compiled by the GBS-CIDP foundation [15] can be helpful resources when considering potential aids.
Performance of daily activities should be regularly monitored and evaluated. As the person regains strength and activity tolerance, task or environmental adaptations should be graded to encourage further independence and participation (Images 6-8). An increasing number and complexity of activities can then be introduced such as work and hobbies (Images 9-10).
Rehabilitation of mobility should proceed concurrently, also using an adaptive approach. A strengthening programme can initially be performed in gravity-eliminated positions and/or utilise adjuncts such as de-weighting equipment. Once able, this should be progressed to gravity-dependent positions using equipment such as a tilt table, electric standing frame and standing hoist and then take the form of functional tasks. Mobility aids and/or orthotics (Table 1) can be considered to enable earlier participation in transfers and mobility and to promote independence.
Restoration
As nerve recovery continues, the rehabilitation approach should focus on restoration, with the goal of returning to pre-disease function and mobility levels. Therapists should aim to reduce environmental and task adaptations, promoting the recovery of underlying impairments. However, some adaptive and preventative approaches may need to continue longer-term, particularly in those with axonal damage. Rehabilitation programmes should aim to address loss of cardiovascular fitness which may be profound, particularly in those that required a prolonged hospital stay.
People with GBS need to be supported to transition from an inpatient rehabilitation setting to the community. Care and time are required to support people to adjust to living with ongoing physical dependence. Education, coaching, connecting with peers and accessing charity support (e.g. GAIN and GBS CIDP Foundation [11,15]) can all support with this difficult transition.
As many as 14% of people with GBS experience moderate-to-severe residual disability. At 10 years, limitations to walking, functional use of the arms, facial weakness and paraesthesia can persist [16]. People may make substantial changes to their job, hobbies and role within their family and reduce their participation in leisure and social activities [17]. Ongoing access to community and/or outpatient services is therefore vital but unfortunately rarely available over prolonged periods. This may include specialist services such as orthotics, vocational rehabilitation and driving assessment centres.
Education, adjustment and psychological support
Formal neuropsychology input is often required for what is effectively a traumatic sequence of events, particularly in those with more severe impairment. It is essential to monitor mood regularly, and to involve psychology or psychiatry colleagues early, as appropriate. The MDT should be mindful that people with GBS are often unable to access specific peer support. There is a lack of GBS specialist centres (unlike spinal cord injury and stroke units) and therefore people are admitted to rehabilitation units with those who have very different conditions and needs.
Discussions about prognosis should ideally happen early in the course of the disease to support with adjustment; however optimal timing of these discussions will vary depending on individual circumstances. The MDT should be realistic, considering prognostic factors [7,18] but aware of the impact this information could have on the person’s mood and engagement in rehabilitation. In more severe cases, there may be loss of hope. Coaching and education play important roles, alongside regular collaborative goal setting that allows frequent conversation regarding prognosis, recovery and function.
Pain management
Pain is a common symptom of all variants of GBS, occurring in up to 89% of people during the course of the disease [19]. Intensity can be moderate to severe and may persist one year after GBS onset [20]. Neuropathic pain is associated with reduced quality of life [21] and often affects participation in rehabilitation. Furthermore, the presence of allodynia and/or hypersensitivity may reduce tolerance to carer handling and use of positioning aids and splints. Pain management should therefore be of highest priority, but it may be complex as neuropathic pain often co-exists with nociceptive pain [7]. We recommend careful MDT assessment of pain and the early aggressive up-titration of neuropathic agents, opiates and/or non-steroidal medications as tolerated and as appropriate. Involvement of a specialist pain team and psychology colleagues can be hugely valuable in successfully managing pain.
Fatigue
Severe fatigue affects 60% of people with GBS [22]. Fatigue reduces the person’s capacity to engage in rehabilitation and participate in daily activities including socialising with family and friends. It can have a profound impact on quality of life. Fatigue requires active management throughout all phases of recovery employing principles that are applicable to other neurological conditions. This should include a coordinated effort across the MDT; planning the day to balance activity and rest times, pacing and modifying tasks. Education is key in supporting the person to make adjustments to their day, which can be frustrating when this includes periods of rest to avoid a ‘boom and bust’ cycle.
Conclusion
The rehabilitation of people with GBS requires a holistic MDT approach to acknowledge and manage a wide variety of symptoms and secondary issues related to the diagnosis. Rehabilitation should consist of three processes: prevention, adaptation, and restoration (Figure 1). Early intervention to prevent loss of joint range of movement by establishing a 24-hour positioning programme is essential. Careful consideration must be made to allow the person to interact with their environment, social networks and interests in order to support their adjustment throughout the course of rehabilitation. Therapists can enable this through adaptation of the task or environment. As recovery continues, rehabilitation should evolve to focus on restoration, aiming for pre-disease levels of function and mobility. There are likely to be several factors that influence a person’s recovery such as fatigue and pain that should be considered and managed at each stage. Similar rehabilitation principles apply to other severe axonal neuropathies such as critical illness neuromyopathy and rarer diseases such as porphyria and tyrosinaemia.
References
- Bragazzi NL, Kolahi AA, Nejadghaderi SA, Lochner P, Brigo F, Naldi A, Lanteri P, Garbarino S, Sullman MJM, Dai H, Wu J, Kong JD, Jahrami H, Sohrabi MR, Safiri S. Global, regional, and national burden of Guillain-Barré syndrome and its underlying causes from 1990 to 2019. J Neuroinflammation. 2021;11:18(1):264. https://doi.org/10.1186/s12974-021-02319-4 https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-021-02319-4
- Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;13;388:717-27. https://doi.org/10.1016/S0140-6736(16)00339-1 https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)00339-1/fulltext
- Shang P, Zhu M, Baker M, Feng J, Zhou C, Zhang HL. Mechanical ventilation in Guillain-Barré syndrome. Expert Rev Clin Immunol. 2020;16(11):1053-1064. https://doi.org/10.1080/1744666X.2021.1840355
- Walgaard C, Lingsma HF, Ruts L, van Doorn PA, Steyerberg EW, Jacobs BC. Early recognition of poor prognosis in Guillain-Barré syndrome. Neurology. 2011;15;76(11):968-75.https://doi.org/10.1212/WNL.0b013e3182104407 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3059137/
- Frenzen PD. Economic cost of Guillain-Barré syndrome in the United States. Neurology 2008; 1;71(1):21-7. https://doi.org/10.1212/01.wnl.0000316393.54258.d1
- Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, Cornblath DR, van Doorn PA, Dourado ME, Hughes RAC, Islam B, Kusunoki S, Pardo CA, Reisin R, Sejvar JJ, Shahrizaila N, Soares C, Umapathi T, Wang Y, Yiu EM, Willison HJ, Jacobs BC. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol 2019; 15(11):671-683. https://doi.org/10.1038/s41582-019-0250-9 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6821638/
- Van Doorn PA, Van den Bergh PYK, Hadden RDM, Avau B, Vankrunkelsven P, Attarian S, Blomkwist-Markens PH, Cornblath DR, Goedee HS, Harbo T, Jacobs BC, Kusunoki S, Lehmann HC, Lewis RA, Lunn MP, Nobile-Orazio E, Querol L, Rajabally YA, Umapathi T, Topaloglu HA, Willison HJ. European Academy of Neurology/Peripheral Nerve Society Guideline on diagnosis and treatment of Guillain-Barré syndrome. Eur J Neurol. 2023;30(12):3646-3674. https://doi.org/10.1111/ene.16073
- Meythaler JM. Rehabilitation of Guillain-Barré syndrome. Arch Phys Med Rehabil. 1997 Aug;78(8):872-9. https://doi.org/10.1016/S0003-9993(97)90203-3
- Sulaiman W, Gordon T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: from bench top research to bedside application. Ochsner J 2013; 13(1):100-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3603172/
- Khan F, Pallant JF, Amatya B, Ng L, Gorelik A, Brand C. Outcomes of high- and low-intensity rehabilitation programme for persons in chronic phase after Guillain-Barré syndrome: a randomized controlled trial. J Rehabil Med 2011; 43(7):638-46. https://doi.org/10.2340/16501977-0826
- GAIN charity https://gaincharity.org.uk
- Ability net charity https://www.abilitynet.org.uk/
- Buschette S, Kullot J, McGauvran A. Evidence of Occupation-Based Interventions for Acute Inflammatory Demyelinating Polyneuropathy Symptoms: A Critically Appraised Topic. Critically Appraised Topics 2020. https://commons.und.edu/cat-papers/2
- Living Made Easy: https://www.livingmadeeasy.org.uk
- GBS CIDP Foundation: https://www.gbs-cidp.org
- Forsberg A, Press R, Holmqvist LW. Residual disability 10 years after falling ill in Guillain-Barré syndrome: a prospective follow-up study. J Neurol Sci. 2012;15;317(1-2):74-9. https://doi.org/10.1016/j.jns.2012.02.026
- Bernsen RA, de Jager AE, Schmitz PI, van der Meché FG. Long-term impact on work and private life after Guillain-Barré syndrome. J Neurol Sci 2002; 15;201(1-2):13-17. https://doi.org/10.1016/S0022-510X(02)00158-2
- Doets AY, Lingsma HF, Walgaard C, Islam B, Papri N, Davidson A, Yamagishi Y, Kusunoki S, Dimachkie MM, Waheed W, Kolb N, Islam Z, Mohammad QD, Harbo T, Sindrup SH, Chavada G, Willison HJ, Casasnovas C, Bateman K, Miller JAL, van den Berg B, Verboon C, Roodbol J, Leonhard SE, Benedetti L, Kuwabara S, Van den Bergh P, Monges S, Marfia GA, Shahrizaila N, Galassi G, Péréon Y, Bürmann J, Kuitwaard K, Kleyweg RP, Marchesoni C, Sedano Tous MJ, Querol L, Illa I, Wang Y, Nobile-Orazio E, Rinaldi S, Schenone A, Pardo J, Vermeij FH, Lehmann HC, Granit V, Cavaletti G, Gutiérrez-Gutiérrez G, Barroso FA, Visser LH, Katzberg HD, Dardiotis E, Attarian S, van der Kooi AJ, Eftimov F, Wirtz PW, Samijn JPA, Gilhuis HJ, Hadden RDM, Holt JKL, Sheikh KA, Karafiath S, Vytopil M, Antonini G, Feasby TE, Faber CG, Gijsbers CJ, Busby M, Roberts RC, Silvestri NJ, Fazio R, van Dijk GW, Garssen MPJ, Straathof CSM, Gorson KC, Jacobs BC; IGOS Consortium. Predicting Outcome in Guillain-Barré Syndrome: International Validation of the Modified Erasmus GBS Outcome Score. Neurology. 2022;98(5):e518-e532. https://doi.org/10.1212/WNL.0000000000013139
- Moulin DE, Hagen N, Feasby TE, Amireh R, Hahn A. Pain in Guillain-Barré syndrome. Neurology 1997; 48(2):328-31. https://doi.org/10.1212/WNL.48.2.328
- Ruts L, Drenthen J, Jongen JL, Hop WC, Visser GH, Jacobs BC, van Doorn PA; Dutch GBS Study Group. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology 2010; 19;75(16):1439-47. https://doi.org/10.1212/WNL.0b013e3181f88345
- Swami T, Khanna M, Gupta A, Prakash NB. Neuropathic Pain in Guillain-Barré Syndrome: Association with Rehabilitation Outcomes and Quality of Life. Ann Indian Acad Neurol 2021; 24(5):708-714. https://doi.org/10.4103/aian.AIAN_602_20 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8680908/
- Garssen MP, Van Koningsveld R, Van Doorn PA. Residual fatigue is independent of antecedent events and disease severity in Guillain-Barré syndrome. J Neurol. 2006 Sep;253(9):1143-6. https://doi.org/10.1007/s00415-006-0163-6 Epub 2006 Sep 22. PMID: 16998652.




