Abstract
A 77-year-old woman presenting with severe acute ischaemic stroke failed a swallow screening test on admission. A nasogastric (NG) tube was inserted to initiate enteral feeding. She required multiple NG tube placements due to agitation with repeat malposition into the right bronchus demonstrated on chest radiographs. Further radiographs to confirm NG tube position showed a right apical pneumothorax that was missed on initial imaging reviews. The patient remained clinically stable with no respiratory compromise and the pneumothorax resolved spontaneously without directed treatment.
Key Points
- Thoracic complications, of which pneumothorax is the most common, can occur after inadvertent malposition of nasogastric tubes into the tracheobronchial tree
- Chest x-rays to confirm for nasogastric tube position should always be evaluated for pulmonary complications, especially in the context of recent malposition
- Challenging or multiple nasogastric tube placements confer an increased risk of tracheobronchial complications
Background
Nasogastric tubes play a vital role in the nutritional support of patients with acute dysphagia or decreased level of consciousness following acute stroke. Though generally seen as safe, the procedure of nasogastric tube insertion is not without risks. We present the case of a pneumothorax following repeated nasogastric tube insertion into the right tracheobronchial tree.
Case Presentation
A 77-year old woman with a history of hypertension was admitted to hospital after presenting with vomiting, aphasia and right-sided weakness. CT head showed an acute left middle cerebral artery (MCA) territory infarction, with suspicion of a thrombus within the M2 branches of the left MCA. National Institutes of Health Stroke Scale score on presentation to the Emergency Department was 28, but the patient was not eligible for acute reperfusion therapy due to wake-up stroke. She failed a swallow screening test on admission and a nasogastric (NG) tube was inserted to initiate emergency enteral feeding.
Her early nutritional management was complicated by repeated NG tube displacement or removal due to agitation, despite the use of safety mittens and a nasal bridle to mitigate this risk. Seven NG tube reinsertions were required within the first eight days of her admission. On days six and eight of the admission, two separate chest radiographs to check NG tube placement were performed; these demonstrated malposition into the right main bronchus and over the right lung field (Figures 1 and 2). On both radiographs, there was no obvious pneumothorax present.
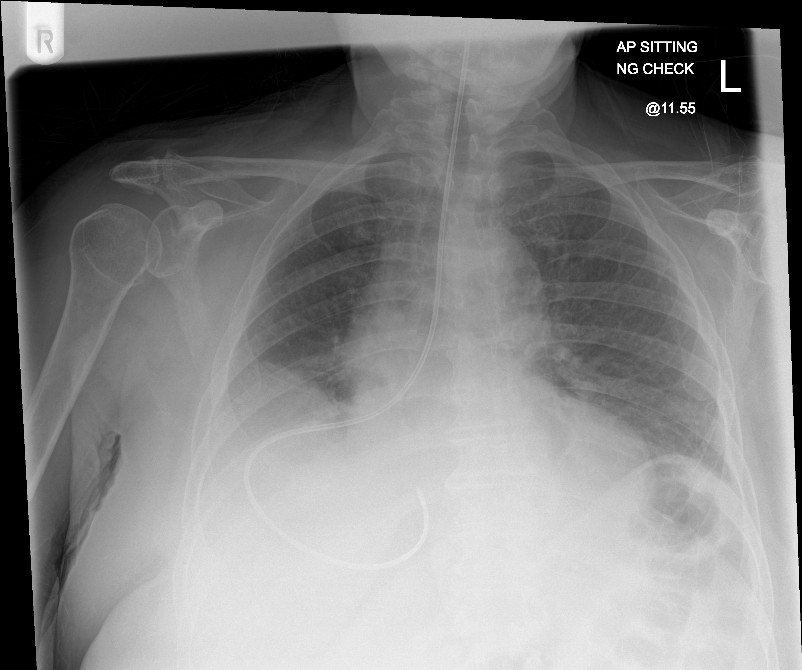
Figure 1: Chest radiograph showing passage of NG tube into the right main bronchus
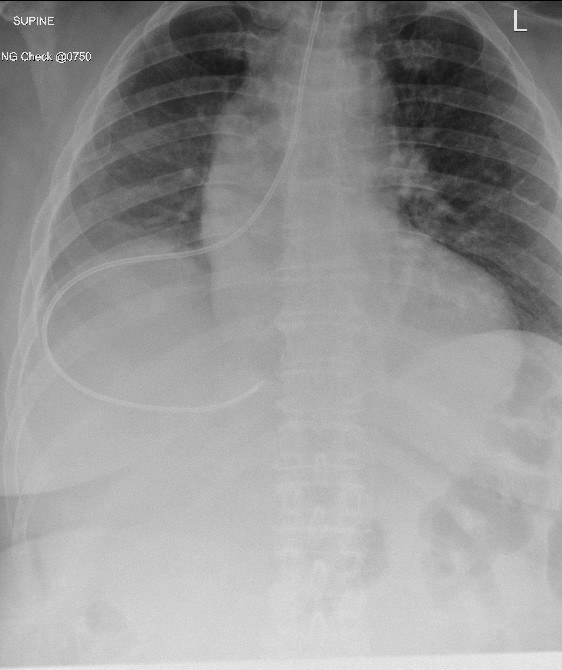
Figure 2: Second malpositioned NG tube insertion into the right lung
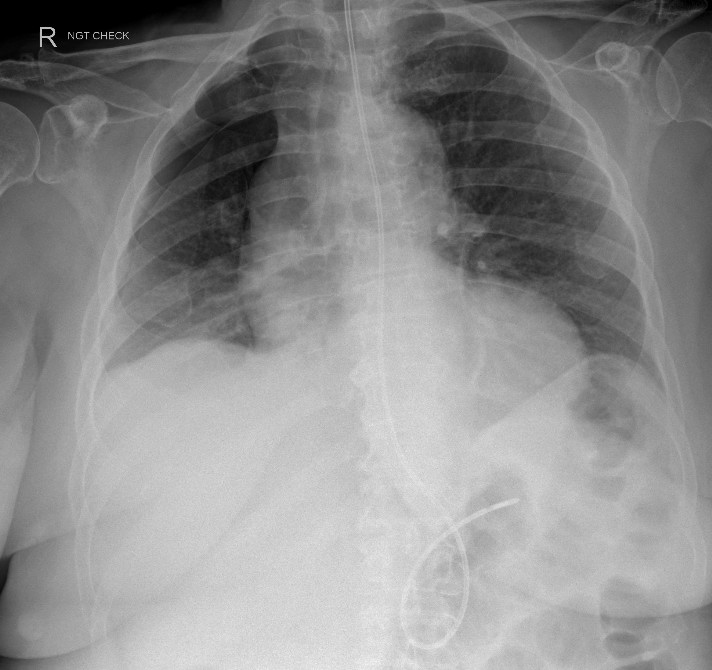
Figure 3: Right pneumothorax
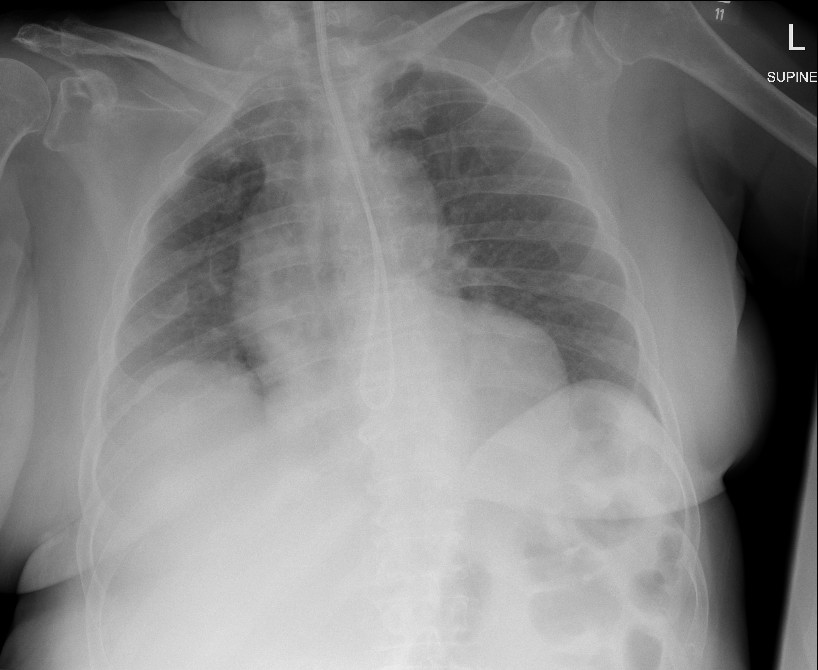
Figure 4: Spontaneous resolution of pneumothorax
A subsequent chest radiograph to check NG tube placement on day nine showed a new right apical pneumothorax (Figure 3). The NG tube was deemed safe to use but no acknowledgement of the pneumothorax was made in the radiograph report or the patient’s clinical notes.
Two days later, after a further chest radiograph was performed to confirm NG tube placement, the on-call radiologist contacted the treating medical team to inform them of the right apical pneumothorax, which had persisted on sequential radiographs. The patient was re-reviewed clinically and found to have reduced air entry on chest auscultation of the right upper zone. She remained stable with no respiratory symptoms and maintained target oxygen saturations (94-98%) on room air. The patient was observed, and subsequent chest radiograph two days after the initial radiological finding showed spontaneous resolution of the pneumothorax (Figure 4).
The patient was 146cm tall, weighing 71.6kg, with a body mass index of 33.5kg/m2. She did not fit the archetypal patient (namely “tall, thin, young and male”) that might present with primary spontaneous pneumothorax (PSP), nor was there a positive family history of PSP. She was a never-smoker with no known underlying lung conditions. The treating team considered performing a CT thorax to screen for secondary causes of spontaneous pneumothorax – but after discussion with the radiology team this was deemed not necessary, with the probable aetiology being iatrogenic following NG tube misplacement.
The patient suffered no complications from the event and made a full recovery. She was successfully switched to oral feeding after a videofluoroscopy swallow study (VFSS).
Discussion
Nasogastric tube insertion is a common but invasive bedside procedure for hospitalised patients, and insertion is usually performed blindly without endoscopic or radiographic guidance. The first-line method for NG tube placement confirmation, recommended by the National Institute for Health and Care Excellence, is pH testing of NG tube aspirates [1]. However, chest radiographs, which are considered the gold-standard for confirming proper position, are often obtained if this is unsuccessful. Chest radiographs must be interpreted carefully in this context and a four-point algorithm is often used to confirm correct NG tube position [1].
In the case we have presented, the right apical pneumothorax was missed on initial reviews on two separate radiographs by both the treating team and reporting clinicians. The underlying causes of such ‘perceptual’ errors, which occur during the initial phase of image interpretation, have been explored by Bruno et al. and include poor conspicuousness of the abnormality, environmental distractions and reader fatigue [2]. The ‘satisfaction of search’ error, where the interpreter fails to continue to search for additional findings after identifying an initial one, may have also played a role here; with an attentional bias towards checking for NG tube position, once this was confirmed the interpreting clinicians may have been less conscientious of other radiographical abnormalities.
Studies have reported the incidence of inadvertent malposition of NG tubes into the tracheobronchial tree to be 0.3-15% [3, 4]. Thoracic complications include pneumonia, lung abscess, pneumothorax, empyema and pulmonary haemorrhage – of these, pneumothorax is the most common [4]. Patients may not present with symptoms indicative of malposition, but it is important to be mindful of potential pulmonary sequelae, especially in the context of repeated insertion after previous pulmonary misadventure.
The risk of challenging or multiple NG tube placements is increased by patient factors including critical illness, altered mental status, non-cooperativeness and an impaired cough reflex [5] – factors which are not uncommon in the acute stroke patient cohort. Over the first eleven days of the patient’s admission, NG tube malposition was confirmed by chest radiograph on eight occasions. Given the increased risk, we suggest that any future NG tube insertion attempts for this patient should be performed under fluoroscopic guidance, which can provide real-time continuous visualisation of the tube as it passes through the pharynx and oesophagus into the stomach, reducing the risk of placement into the respiratory tree [5]. Another technique to prevent tracheobronchial NG tube insertion is Roubenoff and Ravich’s two-step method [6]. However, it is time-intensive and its need for two radiographs limits its practicality and cost-effectiveness in acute care settings.
Acute stroke patients whose nutrition and medication administration are adversely affected by NG tube problems may also benefit from early VFSS, with the aim of facilitating safe but earlier oral intake and NG tube removal. One study showed VFSS performed within 7 days of stroke onset led to relaxation in feeding restrictions for over a quarter of the patient cohort [7]. Swallow function in the acute stroke phase is dynamic, with the potential for rapid spontaneous recovery.
It is imperative for clinicians to be vigilant of the pulmonary complications of NG tube insertion and scrutinise fully radiographs which may have only been requested to confirm NG tube position.
References
- National Patient Safety Agency guidance (https://www.england.nhs.uk/wp-content/uploads/2016/07/Resource_set_-_Initial_placement_checks_for_NG_tubes_1.pdf)
- Bruno MA, Walker EA, Abujudeh HH. Understanding and confronting our mistakes: the epidemiology of error in radiology and strategies for error reduction. Radiographics. 2015 Oct;35(6):1668-76. https://doi.org/10.1148/rg.2015150023
- Rassias AJ, Ball PA, Corwin HL. A prospective study of tracheopulmonary complications associated with the placement of narrow-bore enteral feeding tubes. Critical Care. 1998 Jun;2(1):1 https://doi.org/10.1186/cc120
- Pillai JB, Vegas A, Brister S. Thoracic complications of nasogastric tube: review of safe practice. Interactive cardiovascular and thoracic surgery. 2005 Oct 1;4(5):429-33. https://doi.org/10.1510/icvts.2005.109488
- Kim J, Shin JH. Placement of feeding tubes using fluoroscopy guidance and over-the-wire technique: A technical review. Gastrointestinal Intervention 2017; 6(2): 135-1395. https://doi.org/10.18528/gii160022
- Roubenoff R, Ravich WJ. Pneumothorax due to nasogastric feeding tubes: report of four cases, review of the literature, and recommendations for prevention. Archives of Internal Medicine. 1989 Jan 1;149(1):184-8. https://doi.org/10.1001/archinte.149.1.184
- Kim et al. Usefulness of early videofluoroscopic swallowing study in acute stroke patients with dysphagia. Annals of Rehabilitation Medicine. 2018 Feb 42(1): 42-51. https://doi.org/10.5535/arm.2018.42.1.42
