Abstract
The recent availability of disease modifying treatments (DMTs) for progressive multiple sclerosis (PMS) is a welcome change, yet the limitations of clinical trial design and the real-world makeup of the PMS population necessitate a balanced view of their potential benefits and risks in a population that is on average older than the relapsing-remitting MS (RRMS) population, and more likely to have or develop comorbidities over time. Here we will review the available data for DMT efficacy and risks in PMS with a view to guiding clinician and patient in joint care decision making.
Introduction
People with RRMS (pwRRMS) comprise the majority of new MS diagnoses, however a significant proportion of pwRRMS go on to develop secondary progressive (SPMS). Early natural history studies suggested this to be as high as 90% of pwRRMS by 25 years, at a conversion rate of approximately 2-3% per year [1,2], though recent studies suggest this might be lower (35-62% by 20 years, up to 75% by 30 years) due to DMT use [3-7] and predicated on other risk factors [8-10]. In addition, 10-15% of new diagnoses consist of primary progressive MS (PPMS) [11]. Considering that the total number of people with MS (pwMS) in the United Kingdom (UK) is approximately 130,000 [12], this means that there are at least 50,000 people with PMS at any one time point [13]. Lastly, with the advent of multiple high efficacy therapies, it’s appreciated that MS exists on a spectrum, with progression occurring independently of relapses, and during the RRMS phase [14-16].
The licensed treatment option in the UK for active PPMS is ocrelizumab [17], and for active SPMS include either siponimod [18] or rarely, Interferon beta-1b (brand name Extavia) [18]. Their initiation requires evidence of MRI or clinical relapse to be eligible. Trial design, however, targets statistical significance for the primary efficacy outcome measure rather than secondary safety analyses, and there is no agreed gold standard definition of a true risk signal from safety monitoring. These issues are compounded by the recruitment of a lower-risk population, inconsistencies in adverse event reporting and misclassification, and lack of generalisability from either the trial population or a restricted dosage regime [19].
This is of salience in PMS which occurs more frequently in an older and more vulnerable population group (mean age of onset is 45 years in SPMS [20], and 40 years in PPMS [21]), as well as the increasing mean life expectancy in MS, from a historic expectancy of 66 years to more recent studies suggesting around 75 years [22-26].
The Importance of Age
Age appears to be a major determinant of DMT effect, a meta-analysis of clinical trial data identified reduced likelihood of efficacy after age 53 in the average pwMS [20]. Compounding this is the potential risk of side effects such as opportunistic infection, malignancy, and autoimmune events, which are more likely with advancing age or greater duration of treatment [27-30].
Immunosenescence is more likely with advancing age, with age-related changes in both adaptive and innate immune cells [31] being seen. This can contribute to the risk of cancer [32], opportunistic infections (including rare cases of progressive multifocal leukoencephalopathy ((PML)), or worse outcomes following infection [22,33-34].
Additional risk arises from other conditions that more commonly develop with age, such as hypertension, diabetes, ocular pathology, and cardiovascular disease. A recent UK-based study simulated evidence that 2/3 of the adult population older than 65 years will be living with multiple comorbidities by 2035 [35].
Beta Interferons (INF-β)
Extavia (interferon β-1b, INF- β-1b) is licensed for SPMS with relapses by NICE [36], however from internal calculations the overall number of pwSPMS on Extavia is likely to be ≤1%. INF- β-1b has been the subject of extensive experience and longitudinal study and has consistently been found to be a safe medication [37-40].
The infection risk is limited, with a minimal demonstrable increased rate of crude infections compared to the general population (incidence rate, 8.9% vs 5.2% per 1000 person years) [41]. It is not associated with opportunistic infections [42], and the only reported case of PML with interferon monotherapy occurred in an individual with common variable immunodeficiency syndrome [43].
Within one observational study over 12 years [40], a non-significant trend towards risk of breast cancer cases in those treated with INF-β (OR 1.77) was seen – however without a dose-response effect or discrepancies in tumour size. Another, smaller study from Israel of 1,338 pwMS demonstrated a borderline association with non-breast cancer risk that did not reach statistical significance [40].
Larger studies, including a French study involving 12 MS centres, revealed no increased risk of cancer with any IFN-β exposure [44], supported by post-marketing industry-sponsored studies of insurance claims showing no increase in cancer rates – however both were over a brief 2–3-year period only [45-46].
The efficacy of continued interferon use is debatable; an Italian study [47] demonstrated that of an SPMS cohort, divided into two groups, one continuing treatment for a minimum of 36 months, and the other stopping, there was no difference in accrual of an extended disability score (EDSS) of 7.0 over a 10-year period.

Siponimod for SPMS
Siponimod rapidly depletes T lymphocytes from the peripheral circulation by sequestering them within lymphoid tissue, thereby preventing them from migrating to the central nervous system (CNS), and potential further impact on CNS cells [48]. Siponimod acts only on sphingosine-1-phosphate-receptors 1 and 5, reducing the risk of adverse effects [49-50].
The EXPAND trial demonstrated siponimod’s efficacy in cases of active SPMS in 2018, with a significant reduction in 3-month confirmed disability progression (CDP) (21% reduced HR), and subgroup analysis demonstrating a marked reduction in both 3-month CDP (36% reduced HR) and 6-month CDP (41% reduced HR) versus placebo [51]. Its side effect profile is well documented and summarised in Table 2.
A German retrospective multi-site observational study [50] of 227 pwSPMS over an 18-month period, supported the benefits of siponimod. At 12 months, almost 65% had experienced disease stability (and improvement in 21.4%).
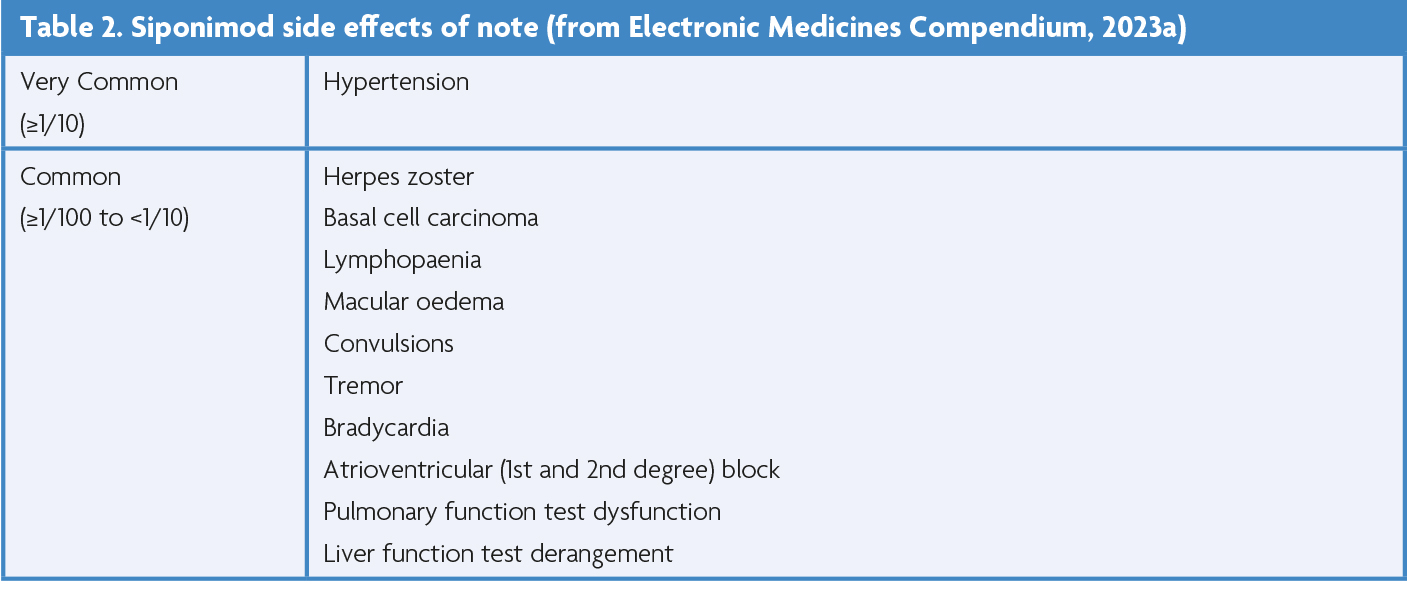
EXPAND highlighted infection as a significant complication of siponimod vs placebo, specifically for varicella zoster virus (VZV) reactivation (2% vs 1%) and herpes infection (5% vs 3%), including one case of herpes zoster (HZ) meningitis. In the context of age and age-related co-morbidities, 68% of VZV infections in the general population occur after the age of 50 [49,50], with relative risk of infection increasing by 1.65 times after the age of 60. A variety of comorbidities (including diabetes, cardiovascular and renal disease, and rheumatoid arthritis) contribute to this risk further (RR range, 2.08-1.23) [53].
COVID-19 related data has been encouraging, with evidence that siponimod use doesn’t predispose to higher risk of severe outcomes [54], however it does impair the humoral vaccine response [55,56].
PML has been reported in 3 cases, one in the EXPAND trial extension, and two in post-marketing. Two have been attributed to siponimod directly, in a 63-year-old male and 62-year-old female, with the duration of use being 6.5 years and 8 years respectively [57].
Though skin cancer rates in the EXPAND study were similar between cases and controls (all skin neoplasms n=14/1099 vs n=8/546, and BCC n=11/1099 vs n=6/546, respectively), it raised concern of potentially increased skin cancer risk [51]. A recent real-world study utilising the FDA adverse event reporting system, showed patients on siponimod were 11.32 times more likely to develop skin cancer (crude reported odds ratio). On further sensitivity analysis, basal cell carcinoma (BCC) was 22.83 times more likely to occur in the treatment group vs placebo [58].
Significant lymphopaenia did not appear to be a major adverse event in the original study [51], with only 1% of participants experiencing a grade IV lymphopaenia (absolute lymphocyte count <200cells/mm¬¬3), and normalisation occurring within 2 months of discontinuation [48]. The German group’s findings support this, with lymphopaenia affecting 38.1% of their enrolled participants, however only resulting in treatment discontinuation in a minority [50].
The long-term development of hypertension in an older population with siponimod use, occurred in 16.2% of the 227 pwSPMS in the German study [50], and potentially greater risk of macular oedema in the context of diabetes, uveitis, or other underlying retinal disease [52].
The cardiac safety profile of siponimod from EXPAND was favourable compared to fingolimod [51], with only a small mean decrease in heart rate (by 3.1 beats/minute) by 7 days being seen, and no second- or third-degree heart block on telemetry lasting up to 6 days.
The AMASIA study is a German prospective non-interventional observational study, assessing the long-term effectiveness and safety of siponimod in routine clinical use for SPMS. It is running across 250 sites, was initiated in 2020, and is due to conclude in 2025 [59]. Ultimately, though it is unclear when siponimod should be discontinued, the Canadian agency for drugs and technologies in health recommends discontinuation if the EDSS reaches 7 (i.e., being wheelchair bound), or if there is a worsening of timed-25-foot walk of ≥20% while on siponimod [60].
As lymphopaenia was the most common side effect [50], it’s important to be aware of the recommended management steps; should an absolute lymphocyte count drop below 0.2 x 109/l, the dose should be reduced from 2mg to 1mg, and if persistent, treatment should be interrupted until counts recover to 0.6 x 109/l before considering re-initiation [52]. The management of hypertension should also be considered, but broadly speaking this would involve weighing a risk/benefit decision regarding continuing siponimod, and then (via GP) typically initiating either an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker in those under the age of 55 years, or a calcium channel blocker in those aged 55 or over or of African/Caribbean descent [61].
Ocrelizumab for PPMS
Ocrelizumab is a humanised anti-CD20 antibody that depletes mature and immature B cells, while sparing long-lived CD20-negative plasma cells [62].
The ORATORIO trial demonstrated efficacy in active primary progressive MS in 2017 [63], reducing rates of 12-week CDP over a 120-week period against placebo (24% reduced HR), with subgroup analyses supporting its benefit on 12-week CDP in patients with gadolinium-positive scans at baseline (35% reduced HR), resulting in approval for its use in the UK in 2019 [64]. The risk profile is clearly described (highlighted in Table 3) and is shared with B-cell depleting therapies (BCDTs) [65].
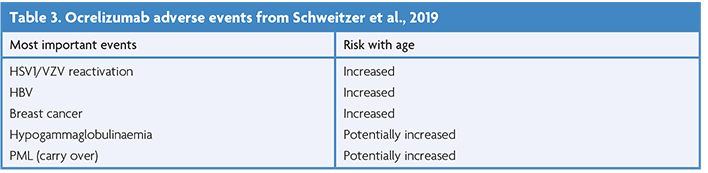
Studies have suggested that being ≥60 years old confers greater risk of hypogammaglobulinaemia, neutropaenia, and infections generally [66,67]. Concerns of immunosuppression have been highlighted by case reports of severe infections in patients over the age of 70 with rituximab related hypogammaglobulinaemia that could not be controlled with antimicrobial therapy [68], increased rates of herpetic reactivation [69], and loss of historic immunity to VZV [70]. This is supported by data demonstrating a greater risk of severe COVID19 outcomes in pwMS on ocrelizumab, as well as older ages, males with comorbidities, greater disability, and a longer duration of MS diagnosis [71,72]. Similarly, the use of ocrelizumab has been found to result in lower seroconversion and humoral immunity response rates following COVID19 vaccination [55,73].
There have been 12 reported cases of PML in pwMS while on ocrelizumab (reflecting 0.00005% of the worldwide population on ocrelizumab, or 1/20, 833 cases) [74,75], 10 of which were attributed to a cross-over effect, having occurred up to several months following conversion from a previous drug that was known to increase the risk of PML, with similar findings in rituximab [30]. The remaining two cases had no history of immunosuppression or use of immunosuppressants; one patient was in their fifties, and the other in their seventies with an underlying immunosenescence and low pre-ocrelizumab lymphocytes count. Ultimately both individuals died from PML-related complications [74].
The ORATORIO trial demonstrated a non-significant increase in the number of malignancies in patients treated with ocrelizumab (11/486 cases, 2.3%, versus placebo 2/239 cases, 0.84%) [63]. Assessment of all trial data by Genetech of the breast cancer risk also shows a non-insignificant increase in rates of females treated with ocrelizumab (6/781, 0.77%, versus 0/668 controls treated with Rebif or placebo [74]. However, the rate was within the background rate expected for an MS population, which is important to consider in the context of individual cancer risks. Similarly, BCC incidence appeared to be greater between years 3-4 of treatment, but this was not sustained in subsequent years and again was in keeping with background MS rates (Schweitzer et al., 2019; Electronic Medicines Compendium, 2023).
A large German prospective non-interventional observational study, CONFIDENCE, for 3,000 RRMS and PPMS treated with ocrelizumab launched in 2020 and will provide significant long-term real world safety data [77].
Alternative and Emerging Potential Treatments
Treatment regulation varies between countries; it is helpful to be aware that most treatments available for RRMS are also options in active PMS in other countries, such as the United States of America (USA) [78]. Among those is the Federal Drug Association (FDA) licensed Ofatumamab, a fully human anti-CD20 monoclonal antibody and BCDT [79]. The phase 3 ASCLEPIOS I and II trials involved both pwRRMS and active SPMS, and showed, compared to Teriflunomide, a reduction in annualised relapse rates (0.11 versus 0.22) and lower 3-month CDP (HR 0.68) [80], with only a limited increase in serious infections (2.5% versus 1.8%) [80], and sustained safety evidence in the 4-year ALITHIOS study and phase 2 MIRROR study in pwRRMS [81]. Specifically, the ALITHIOS study showed no increase in infection rates by exposure duration, episodes of opportunistic infection, hepatitis B reactivation, or PML [82,83]. Similarly, there was no evidence of increased neoplasm rates, or clustering of malignancies in the original study, and the follow up 4-year safety data identified malignancies in 11 patients (0.6%), with no increase in incidence rates over time of exposure, with the only clustering being of BCC (n=4) and invasive breast carcinoma (n=2) [82,83].
Bruton Tyrosine Kinase Inhibitors (BTKi) are a novel drug class of small molecules capable of crossing the blood brain barrier, that have the potential to target both the adaptive and innate immune mechanisms of both the peripheral and central nervous system [84]. Multiple agents are undergoing phase 3 trials currently, however phase II and extension safety data has been largely reassuring with the most common reported events being upper respiratory tract infections, headache, and raised liver enzymes [84].
The MS-STAT2 trial, investigating the effect of high-dose (80mg) simvastatin in pwSPMS is due to conclude in late-2024 [85]. Simvastatin is a 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitor, with PMS-relevant properties [86], and directly effects vascular co-morbidity, which has been shown to influence PMS outcomes [87]. Its benefit and safety profiles are well recognised from its common use in vascular diseases [88], which has been mirrored in safety data from the MS-STAT1 trial [89].
Lastly, an in depth summary of the trials landscape in progressive MS has recently been published, which further details the above alongside other completed and ongoing PMS trials [90].
Select Treatment Considerations
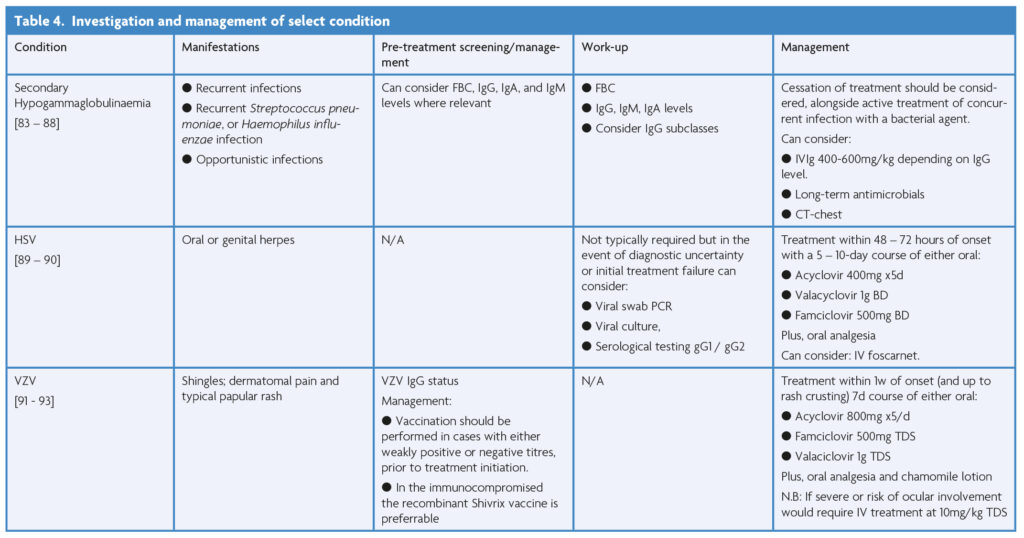
Among the described treatment options certain complications arise with greater frequency and therefore prophylactic considerations or management is worth elaborating on; this is summarised in Table 4 below.
There has been an intense development in our understanding of vaccination success in patients with MS on DMTs since the COVID19 pandemic, it is worth noting the importance of seasonal influenza vaccination generally, and the administration of the 23-valent pneumococcal polysaccharide vaccine (PPV23), in those on long-term immunosuppressive therapy. PPV23 should ideally be administered at least 2 weeks before initiation of maintenance immunosuppressives, and is also recommended for those established on treatment [91,92]. In those already established on ocrelizumab, which can particularly impair the humoral response, antibody titers can be considered to assess whether repeat vaccination is required [93].
Conclusion
In summary, the use of INF- β-1b in relapsing PMS has the most limited risk profile, without convincing evidence of significant adverse effects generally, or infections/cancer specifically – however the evidence of gain from a clinical progression viewpoint is limited, and in the UK is rarely used.
When prescribing siponimod it is important to primarily weigh up the potentially increased risks of herpetic reactions, reduced vaccination efficacy, BCC, hypertension, and macular oedema, in the context of age-related risks. The risk profile for ocrelizumab is greater, with more risk of immunocompromise, severe infection outcomes, impaired vaccine responses and the potential to lose historic immunity, however the cancer risk is less convincing at present and requires a more nuanced approach to an individual’s history.
Ultimately, larger prospective observational data, such as from AMASIA for siponimod and CONFIDENCE for ocrelizumab, are needed to better guide decision making, with planned completion in 2025 and 2028 respectively. In the interim, an open discussion about the above potential benefits, reduced likelihood of DMT impact, and shift in risk profiles with advancing age, needs to be had in order to reach a care decision that takes into consideration an individual’s views and opinions on the potential risks and benefits of continued treatment.
References
- Weinshenker BG et al. The Natural History of Multiple Sclerosis: A Geographically Based Study I. Clinical Course and Disability. 1989. https://doi.org/10.1093/brain/112.1.133
- Barzegar M, Najdaghi S, Afshari-Safavi A, Nehzat N, Mirmosayyeb O, Shaygannejad V. Early predictors of conversion to secondary progressive multiple sclerosis. Mult Scler Relat Disord. 2021;54. https://doi.org/10.1016/j.msard.2021.103115
- Tedeholm H et al. Effectiveness of first generation disease-modifying therapy to prevent conversion to secondary progressive multiple sclerosis. Mult Scler Relat Disord. 2022;68:104220 https://doi.org/10.1016/j.msard.2022.104220
- Scalfari A, Neuhaus A, Daumer M, Ebers GC, Muraro PA. Age and disability accumulation in multiple sclerosis. Neurology. 2011;77(13):1246-1252. https://doi.org/10.1212/WNL.0b013e318230a17d
- Fambiatos A et al. Risk of secondary progressive multiple sclerosis: A longitudinal study. Multiple Sclerosis Journal. 2020;26(1):79-90. https://doi.org/10.1177/1352458519868990
- Coret F et al. Onset of secondary progressive multiple sclerosis is not influenced by current relapsing multiple sclerosis therapies. Mult Scler J Exp Transl Clin. 2018;4(2)205521731878334.https://doi.org/10.1177/2055217318783347
- Cree BAC et al. Long‐term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80(4):499-510. https://doi.org/10.1002/ana.24747
- Barzegar M, Shaygannejad V, Mirmosayyeb O, Afshari A. Progression to Secondary Progressive Multiple Sclerosis and its Early Risk Factors: A Population-based Study (2171) Neurology. 2020;94(15)Supplement(2171). https://doi.org/10.1212/WNL.94.15_supplement.2171
- Taylor BV. Time to onset of secondary progression as an outcome in MS trials: a new paradigm? J Neurol Neurosurg Psychiatry.2014;85(1)3. https://doi.org/10.1136/jnnp-2012-304611
- Cree BAC et al. Secondary Progressive Multiple Sclerosis. Neurology. 2021;97(8):378-388. https://doi.org/10.1212/WNL.0000000000012323
- Weinshenker BG. Natural History of Multiple Sclerosis. 1994. https://doi.org/10.1002/ana.410360704
- MS Society. MS in the UK Estimates of incidence and prevalence of MS in the UK. 2022.
- Vasanthaprasad V, Khurana V, Vadapalle S, Palace J, Adlard N. Systematic literature review and meta-analysis of the prevalence of secondary progressive multiple sclerosis in the USA, Europe, Canada, Australia, and Brazil. BMC Neurol 2022;22(1) https://doi.org/10.1186/s12883-022-02820-0
- Scalfari A, Neuhaus A, Daumer M, DeLuca GC, Muraro PA, Ebers GC. Early Relapses, Onset of Progression, and Late Outcome in Multiple Sclerosis. JAMA Neurol, 2013;70(2):214 https://doi.org/10.1001/jamaneurol.2013.599
- Portaccio E et al. Progression is independent of relapse activity in early multiple sclerosis: a real-life cohort study. Brain. 2022;145(8):2796-2805. https://doi.org/10.1093/brain/awac111
- Kaufmann M et al. Identification of early neurodegenerative pathways in progressive multiple sclerosis. Nat Neurosci. 2022;25(7):944-955. https://doi.org/10.1038/s41593-022-01097-3
- National Institute for Clinical Excellence (NICE). Ocrelizumab for treating primary progressive multiple sclerosis. 2019. [Online]. Available: http://www.nice.org.uk/guidance/ta585
- National Institute for Clinical Excellence (NICE), Siponimod for treating secondary progressive multiple sclerosis. 2020. [Online]. Available: http://www.nice.org.uk/guidance/ta656
- Singh S, Loke YK. Drug safety assessment in clinical trials: methodological challenges and opportunities. Trials. 2012;13(20).https://doi.org/10.1186/1745-6215-13-138
- Kes VB, Grbić N, Jurašić MJ, Zavoreo I, Matovina LZ. Secondary progressive multiple Sclerosis. Acta Medica Croatica. 2018;72(3):381-384. https://doi.org/10.1212/NXI.0000000000200064
- Tremlett H, Zhao Y. Primary and secondary progressive MS have a similar age at onset of progression – NO. Multiple Sclerosis. 2017;23(5)640-642. https://doi.org/10.1177/1352458516684559
- Mawer C. Data show big rise in deaths of people with neurological disorders – Rapid Response. BMJ. 2018.
- Rollot F et al. Effects of Age and Disease Duration on Excess Mortality in Patients With Multiple Sclerosis From a French Nationwide Cohort. Neurology. 2021;97(4) https://doi.org/10.1212/WNL.0000000000012224
- Willumsen JS, Grytten N, Aarseth J, Myklebust TÅ, Myhr KM, Midgard R, Mortality and cause of death in multiple sclerosis in western Norway 1950-2021: a registry-based linkage study. J Neurol Neurosurg Psychiatry. 2022;93(11):1154-1161. https://doi.org/10.1136/jnnp-2022-329169
- Lunde HMB, Assmus J, Myhr KM, Bø L, Grytten N. Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J Neurol Neurosurg Psychiatry. 2017;88(8):621-625. https://doi.org/10.1136/jnnp-2016-315238
- Leadbetter R, MacAskill M, Myall DJ, Taylor BV, Joshi P, Mason DF. Multiple sclerosis mortality in New Zealand: a nationwide prospective study. J Neurol Neurosurg Psychiatry. 2023;94(7)511-517. https://doi.org/10.1136/jnnp-2022-330582
- Lebrun C, Rocher F. Cancer Risk in Patients with Multiple Sclerosis: Potential Impact of Disease-Modifying Drugs. CNS Drugs.Springer International Publishing. 2018:32(10):939-949. https://doi.org/10.1007/s40263-018-0564-y
- Grebenciucova E, Pruitt A. Infections in Patients Receiving Multiple Sclerosis Disease-Modifying Therapies. Curr Neurol Neurosci Rep. 2017;17(11):88. https://doi.org/10.1007/s11910-017-0800-8
- Förster M et al. Managing Risks with Immune Therapies in Multiple Sclerosis. Drug Saf. 2019;42(5):633-647. https://doi.org/10.1007/s40264-018-0782-8
- Schweitzer F et al. Age and the risks of high-efficacy disease modifying drugs in multiple sclerosis. Current Opinion in Neurology.Lippincott Williams and Wilkins 2019;32(3):305-312. https://doi.org/10.1097/WCO.0000000000000701
- Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24(5):331-341. https://doi.org/10.1016/j.smim.2012.04.008
- Cancer Research UK. Cancer Incidence by Age. https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/age#heading-Zero.
- Gheuens S, Pierone G, Peeters P, Koralnik IJ. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J Neurol Neurosurg Psychiatry. 2010;81(3):247-254. https://doi.org/10.1136/jnnp.2009.187666
- Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. 2021;17(1):37-51. https://doi.org/10.1038/s41582-020-00427-y
- Kingston A, Robinson L, Booth H, Knapp M, Jagger C. Projections of multi-morbidity in the older population in England to 2035: estimates from the Population Ageing and Care Simulation (PACSim) model. Age Ageing. 2018;47(3):374-380. https://doi.org/10.1093/ageing/afx201
- NICE. Beta interferons and glatiramer acetate for treating multiple sclerosis Technology appraisal guidance [TA527]. https://www.nice.org.uk/guidance/ta527/chapter/1-Recommendations. 2018.
- Calabresi PA et al. Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol. 2014;13(7)657-667. https://doi.org/10.1016/S1474-4422(14)70068-7
- Hu X et al. COMPARE: Pharmacokinetic profiles of subcutaneous peginterferon beta-1a and subcutaneous interferon beta-1a over 2 weeks in healthy subjects. Br J Clin Pharmacol. 2016;82(2):380-8. https://doi.org/10.1111/bcp.12968
- Hu X et al. A Novel PEGylated Interferon Beta-1a for Multiple Sclerosis: Safety, Pharmacology, and Biology. The Journal of Clinical Pharmacology. 2012;52(6):798-808. https://doi.org/10.1177/0091270011407068
- Kingwell E, Evans C, Zhu F, Oger J, Hashimoto S, Tremlett H. Assessment of cancer risk with β-interferon treatment for multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85(10):1096-1102. https://doi.org/10.1136/jnnp-2013-307238
- Luna G et al. Infection Risks Among Patients With Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol.2020;77(2):184. https://doi.org/10.1001/jamaneurol.2019.3365
- Winkelmann A, Loebermann M, Reisinger EC, Hartung H-P, Zettl UK. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. 2016;12(4):217-233.https://doi.org/10.1038/nrneurol.2016.21
- Lehmann HC, Krüger K, Fink GR, Schroeter M. Progressive multifocal leukoencephalopathy after interferon beta-1a monotherapy. J Neurol. 2015;262(3):771-773. https://doi.org/10.1007/s00415-014-7620-4
- Lebrun C et al. Cancer and multiple sclerosis in the era of disease-modifying treatments. J Neurol. 2011;258(7):1304-1311. https://doi.org/10.1007/s00415-011-5929-9
- Safran J. Assessment of malignancy risk in patients with multiple sclerosis treated with intramuscular interferon beta-1a: retrospective evaluation using a health insurance claims database and postmarketing surveillance data. Ther Clin Risk Manag. 2012:313 https://doi.org/10.2147/TCRM.S31347
- Sandberg-Wollheim M, Kornmann G, Bischof D, Moraga MS, Hennessy B, Alteri E. The risk of malignancy is not increased in patients with multiple sclerosis treated with subcutaneous interferon beta-1a: analysis of data from clinical trial and post-marketing surveillance settings. Multiple Sclerosis Journal. 2011;17(4):431-440. https://doi.org/10.1177/1352458511403642
- Zanghì A, D’amico E, Patti F, Avolio C. Stopping Interferon Beta 1b Does Not Influence the Risk of Disability Accrual in Non-Active SPMS: Results from an Italian Real-World Study. Int J Environ Res Public Health. 2022;19(10). https://doi.org/10.3390/ijerph19106069
- Sabsabi S, Mikhael E, Jalkh G, Macaron G, Rensel M. Clinical Evaluation of Siponimod for the Treatment of Secondary Progressive Multiple Sclerosis: Pathophysiology, Efficacy, Safety, Patient Acceptability and Adherence. Patient Preference and Adherence. Dove Medical Press Ltd. 2022;16:1307-1319. https://doi.org/10.2147/PPA.S221882
- Chaudhry BZ, Cohen JA, Conway DS. Sphingosine 1-Phosphate Receptor Modulators for the Treatment of Multiple Sclerosis. Neurotherapeutics. 2017;14(4)859-873. https://doi.org/10.1007/s13311-017-0565-4
- Regner-Nelke L et al. Real-world evidence on siponimod treatment in patients with secondary progressive multiple sclerosis. Neurol Res Pract. 2022;4(1). https://doi.org/10.1186/s42466-022-00219-3
- Kappos L, Bar-Or A, Cree BAC. ‘Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 stud Lancet. 2018;391(10127):1263-1273.
- Electronic Medicines Compendium. Mayzent – Summary of Product Characteristics. Accessed 06/2023.
- Marra F, Parhar K, Huang B, Vadlamudi N. Risk Factors for Herpes Zoster Infection: A Meta-Analysis. Open Forum Infect Dis. 2020;7(1). https://doi.org/10.1093/ofid/ofaa005
- Sullivan R et al. COVID-19 Infection in Fingolimod- or Siponimod-Treated Patients. Neurology – Neuroimmunology Neuroinflammation. 2022;9(1)p.e1092. https://doi.org/10.1212/NXI.0000000000001092
- Tallantyre EC et al. COVID-19 Vaccine Response in People with Multiple Sclerosis. Ann Neurol. 2022;91(1):89-100. https://doi.org/10.1002/ana.26251
- Krbot Skorić M, Rogić D, Lapić I, Šegulja D, Habek M, Humoral immune response to COVID-19 vaccines in people with secondary progressive multiple sclerosis treated with siponimod. Mult Scler Relat Disord. 2022;57:p.103435. https://doi.org/10.1016/j.msard.2021.103435
- Novartis. Novartis PML Siponimod Report. Accessed: Jun. 19, 2023. [Online]. Available: http://siponimodinfo.com
- Stamatellos VP, Rigas A, Stamoula E, Lallas A, Papadopoulou A, Papazisis G. S1P receptor modulators in Multiple Sclerosis: Detecting a potential skin cancer safety signal. Mult Scler Relat Disord. 2022;59. https://doi.org/10.1016/j.msard.2022.103681
- Hoffmann O, Schreiber H, Klotz L, Weber M, Weiss C, Ziemssen T. AMASIA study: Real world insights on the impact of early siponimod treatment on SPMS patients in Germany (P8-3.013). in Tuesday, April 25, Lippincott Williams & Wilkins, 2023:4390. https://doi.org/10.1212/WNL.0000000000203952
- CADTH. CADTH Canadian Drug Expert Committee Recommendation: Siponimod (Mayzent – Novartis Pharmaceuticals Canada Inc.)/ Ottawa (ON):2020.
- NICE. Hypertension in adults: diagnosis and management. https://www.nice.org.uk/guidance/ng136.
- Mulero P, Midaglia L, Montalban X. Ocrelizumab: a new milestone in multiple sclerosis therapy. Therapeutic Advances in Neurological Disorders. 2018;11. SAGE Publications Ltd. https://doi.org/10.1177/1756286418773025
- Montalban X, Hauser SL, Kappos L. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3)209-220. https://doi.org/10.1056/NEJMoa1606468
- NICE. Ocrelizumab for treating primary progressive multiple sclerosis. 2019.
- Cannon L, Pan A, Kovalick L, Sarkissian A, Wu EY. Secondary immunodeficiencies and infectious considerations of biologic immunomodulatory therapies. Annals of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. 2023 Jun. 01. https://doi.org/10.1016/j.anai.2023.02.010
- Athni TS, Barmettler S. Hypogammaglobulinemia, late-onset neutropenia, and infections following rituximab. Annals of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology, Jun. 01, 2023.https://doi.org/10.1016/j.anai.2023.01.018
- Tallantyre EC et al. Secondary antibody deficiency: a complication of anti-CD20 therapy for neuroinflammation. J Neurol. 2018;265(5):1115-1122. https://doi.org/10.1007/s00415-018-8812-0
- Sacco KA, Abraham RS. Consequences of B-cell-depleting therapy: Hypogammaglobulinemia and impaired B-cell reconstitution. Immunotherapy.Future Medicine Ltd. 2018;10(8):713-728. https://doi.org/10.2217/imt-2017-0178
- Electronics Medicine Compendium, Ocrevus – Summary of Product Characteristics. 2023.
- Rapisarda L et al. Varicella zoster immunity loss in multiple sclerosis patient treated with ocrelizumab. Clinical Immunology. 2021. https://doi.org/10.1016/j.clim.2020.108554
- Simpson-Yap S et al. Associations of Disease-Modifying Therapies With COVID-19 Severity in Multiple Sclerosis. Neurology. 2021;97(19):e1870-e1885 https://doi.org/10.1212/WNL.0000000000012753
- Sormani MP et al. Disease‐Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann Neurol. 2021;89(4):780-789. https://doi.org/10.1002/ana.26028
- Tortorella C et al. Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients With MS Using Different Disease-Modifying Therapies. Neurology. 2022;98(5):e541-e554. https://doi.org/10.1212/WNL.0000000000013108
- Genetech. Genetech Prescribing Information: Ocrelizumab.
- Genetech. Genentech data on file: March 31, 2022, post-marketing experience; March 2022, clinical trials data cut-off. https://www.ocrelizumabinfo.com/index.html. Accessed August 2023.
- Electronic Medicines Compendium, ‘Product Specifications: Ocrelizumab (Ocrevus)’, vol. 06, 2023.
- Dirks P et al. Design of a non-interventional post-marketing study to assess the long-term safety and effectiveness of ocrelizumab in German real world multiple sclerosis cohorts – The CONFIDENCE study protocol’, BMC Neurol. 2020;20(1) https://doi.org/10.1186/s12883-020-01667-7
- MS Society. Disease Modifying Treatments for Multiple Sclerosis. Accessed September 2023. https://nms2cdn.azureedge.net/cmssite/nationalmssociety/media/msnationalfiles/brochures/brochure-the-ms-disease-modifying-medications.pdf.
- Federal Drug Authority (FDA). FDA Ofatumumab Prescribing Data. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf. 2020.
- Hauser SL et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. New England Journal of Medicine. 2020;383(6):546-557. https://doi.org/10.1056/NEJMoa1917246
- Bar-Or A et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis. Neurology. 2018;90(20):e1805-e1814. https://doi.org/10.1212/WNL.0000000000005516
- Hauser SL et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Multiple Sclerosis Journal. 2022;28(10):1576-1590. https://doi.org/10.1177/13524585221079731
- Cohen J et al. Five-Year Safety of Ofatumumab in People Living With Relapsing Multiple Sclerosis (P8-3.004)’, in Tuesday, April 25, Lippincott Williams & Wilkins. 2023:2942. https://doi.org/10.1212/WNL.0000000000202906
- Krämer J, Bar-Or A, Turner TJ, Wiendl H. Bruton tyrosine kinase inhibitors for multiple sclerosis. Nat Rev Neurol. 2023;19(5):289-304. https://doi.org/10.1038/s41582-023-00800-7
- Williams T et al. 007 The MS-STAT2 trial in secondary progressive MS – study design and update. J Neurol Neurosurg Psychiatry. 2022;93(6):A16.1-A16. https://doi.org/10.1136/jnnp-2022-ABN.46
- Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol. 2006;6(5):358-370. https://doi.org/10.1038/nri1839
- Williams T et al. Cardiovascular risk factors in Secondary Progressive Multiple Sclerosis: a cross‐sectional analysis from the MS‐STAT2 randomised controlled trial. Eur J Neurol. 2023. https://doi.org/10.1111/ene.15924
- Talreja O, Kerndt CC, Cassagnol M. Simvastatin. 2023.
- Chataway J, Schuerer N, Alsanousi A. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014:383(9936):2213-2221. https://doi.org/10.1016/S0140-6736(13)62242-4
- Chataway J, Williams T, Li V, Marrie RA, Ontaneda D, Fox RJ. Clinical trials for progressive multiple sclerosis: progress, new lessons learned, and remaining challenges. Lancet Neurol. 2024;23(3):277-301. https://doi.org/10.1016/S1474-4422(24)00027-9
- Public Health England. Immunisation against infectious disease (The Green Book). The Stationery Office: London. 2017.
- Reyes S, Ramsay M, Ladhani S et al. Protecting people with multiple sclerosis through vaccination. Pract Neurol. 2020:435-445. https://doi.org/10.1136/practneurol-2020-002527
- Bar-Or A et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis. Neurology. 2020;95(14):e1999-e2008. https://doi.org/10.1212/WNL.0000000000010380

