Schwann cells are the glia of the peripheral nervous system, ensheathing and myelinating large axons and grouping smaller diameter axons within Remak bundles. Bi-directional signalling between axons and Schwann cells has long been known to be essential for the development of the peripheral nervous system. More recently, (and the focus of this review) it has been shown that axo-glial signalling in neural injury is essential for effective repair and is distinct from signalling events during development.
Injury to the peripheral nervous system can be caused by many insults, from metabolic diseases such as diabetes, inherited genetic disorders such as Charcot-Marie-Tooth disease (CMT), infectious and inflammatory disorders including Guillan-Barré syndrome and traumatic injury which alone affects up to 300,000 people in Europe per year.1 Traumatic nerve injury in rodents is very commonly used as a model to study the process of peripheral nerve repair and functional recovery. Following an injury to the peripheral nervous system, axon and myelin fragments are broken down by a process termed Wallerian degeneration. Axons then regenerate, are remyelinated and eventually reinnervate target organs. How complete this process is and the extent to which target organs are innervated by the correct axons is related to the degree of functional recovery. Signalling between Schwann cells and axons is essential throughout the phases of this process and disruption of this signalling has severe consequences for nerve repair.
How do Schwann cells respond to nerve injury/support axons following nerve injury?
Schwann cells are essential for nerve repair. Following injury, they re-enter the cell cycle and activate Raf/MEK/ERK signalling.2 This drives their differentiation into a phenotype that actively phagocytoses myelin and axonal fragments, promotes recruitment of macrophages, enhances axon growth, and increases neuronal survival. The alignment of Schwann cells into bands of Büngner guides regenerating axons back to their synaptic targets (Figure 1).3,4 Schwann cells undergo this phenotypic transformation as a direct response to axon derived signals (the identity of which are not established), which trigger a transcriptional program driven by the transcription factor c-Jun. This results in a down regulation of genes that regulate myelination and an upregulation of genes involved in macrophage recruitment, axon growth and survival. If this phenotypic switch is disrupted by inactivating c-Jun in Schwann cells, axon regeneration, functional recovery and neuronal survival is severely impaired following peripheral nerve injury.5 This phenotypic plasticity of Schwann cells may in rare instances be detrimental. Mycobacterium leprae, the causative agent of leprosy utilises this property of Schwann cells. Having infected Schwann cells the bacterium triggers reprogramming of these cells to aid bacterial dissemination.6
As axons regenerate through bands of Büngner, in addition to providing guidance towards their targets, Schwann cells must provide other supportive roles (Figure 1 C).
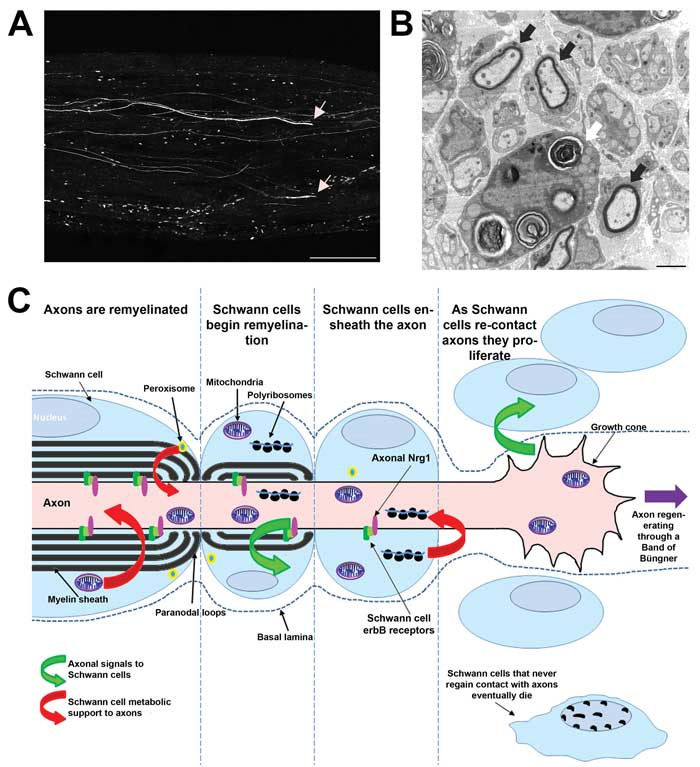
Local translation in axons is likely to be necessary during growth cone formation and axon elongation, indeed, in vitro inhibition of local translation results in retraction of growth cones.7 Polyribosomes have been visualised to transfer from Schwann cells to regenerated axons, raising the possibility that Schwann cells may not only provide translational machinery to support axons but also mRNA and therefore could modify axonal translational products.8
The very long distances between axons and their cell bodies has long implicated a need for Schwann cells to provide a metabolic supportive role.9 In mutant mice in which Schwann cell mitochondria are dysfunctional, development occurs normally, but in adulthood mice develop a severe axonal neuropathy despite axonal mitochondria being unaffected.10 Schwann cell mitochondria function plays a role in repair, as although regeneration is unaffected in mutant mice remyelination fails.10 Furthermore mice with Schwann cells lacking functional peroxisomes, organelles housing oxidative metabolic reactions, present in non-compact myelin membranes, develop an adult-onset neuropathy.11 Recently it has been demonstrated that myelinating Schwann cells contain glycogen granules which are likely to provide a source of glycogen derived lactate to axons in order to metabolically support axons particularly in hypoglycaemic conditions.12 The long narrow channels of glial cytoplasm connecting to the periaxonal space including Cajal bands, Schmidt-Lanterman incisures and the lumina of paranodal loops are likely to provide a means for Schwann cells to transfer metabolites to axons in order to provide metabolic support necessary for maintenance and more than likely essential for the metabolically expensive process of repair of the peripheral nerve.
How do axons regulate SC health and phenotype following nerve injury?
As axons re-contact Schwann cells after nerve injury, signals from the axolemma are critical in directing the differentiation of Schwann cells back into a mature state in which they ensheath and in the case of large diameter axons myelinate axons (Figure 1). There are a few receptor-ligand signalling pairs known to regulate axo-glial signalling, the most characterised of which is the protein Neuregulin-1 type III expressed on the surface of axons which signals through binding to erbB2/erbB3 heterodimer receptors expressed on the Schwann cell. Although essential for peripheral nerve development Neuregulin-1/erbB signalling is dispensable for peripheral nerve maintenance. In contrast, in the early phases following peripheral nerve injury Neuregulin-1/erbB axo-glial signalling drives a transcriptional programme that enhances the rate of remyelination, and regeneration of peripheral axons as well as functional recovery. Interestingly at delayed time points after injury axons remyelinate and function is restored in the absence of axonal Neuregulin-1 implying the presence of alternative signalling systems instructive in determining myelination fate of axons following injury.13,14 It has recently been shown that Schwann cell-derived Neuregulin-1 can also promote remyelination.15 Other axoglial receptor-ligand signalling pairs which are known to regulate myelination during development and potentially mediate nerve repair include axonal adam22 signalling through Schwann cell Lgi4,16 Necl-1 on axons signalling through Schwann cell Necl-417-19 and the as yet unidentified axonal ligand to Schwann cell G-protein-coupled receptor gpr126.20
Ultimately, Schwann cells need axonal contact to survive, this is shown in chronically denervated nerve stumps where, as time progresses, in the absence of axonal contact survival of Schwann cells declines. Importantly the Schwann cells that survive are much less able to support any axons that do eventually regenerate into such a stump.21 This is likely to be caused by transcriptional changes caused by a lack of axo-glial signalling. It is known that the expression of erbB receptors and of the growth factor glial cell-line derived neurotrophic factor (GDNF) is greatly reduced in such chronically denervated Schwann cells.21,22 This decline in Schwann cell capability and survival is clinically very important as the rate of axon regeneration is slow at 1-3mm per day resulting in Schwann cells distal to the injury being denervated for prolonged periods contributing to the poor functional outcomes particularly seen following proximal injuries for instance following brachial plexus avulsion.
We have concentrated on traumatic neuropathy as an exemplar. The critical nature of axo-glial signalling to nerve injury and repair is virtually ubiquitous to all forms of neuropathy. An example is CMT1A which is due to an excessive gene dosage of PMP22 in Schwann cells, resulting in demyelination. However, the level of disability in patients relates to the degree of secondary axonal loss and not the degree of conduction velocity slowing.23
Axo-glial signalling may also be usurped by infective agents. Neuregulin-1 is normally presented to Schwann cells on the axolemma however high doses of exogenous soluble Neuregulin-1 have been reported to cause demyelination by triggering Schwann cell proliferation. The leprosy causing Mycobacterium leprae directly binds to and activates the erbB2 receptor activating the downstream MEK–ERK pathway and causing pathological demyelination.24
Therapeutic opportunities
There is currently no pharmacological intervention to promote peripheral nerve repair. Greater understanding of how Schwann cells provide metabolic and trophic support to axons may provide means to provide axonal protection given that axonal loss is a major determinant of progressive disability. Manipulation of Neuregulin-1/erbB signalling may provide a means to promote remyelination. It is still not clear as to whether administration of exogenous soluble Neuregulin-1 in vivo can substitute for juxtacrine Neuregulin-1 which is presented on the axolemma. A more tractable means of manipulating this pathway may be to inhibit enzymes such as TACE (ADAM17) which process Neuregulin-1 into an inactive form.25 In the problematic situation of chronic denervation substituting signals that Schwann cells would normally receive from axons could promote their survival so that when regenerating axons finally reach distal regions of nerve they enter a much more hospitable environment. Using these approaches we hope that greater knowledge of axo-glial communication can ultimately be used to choreograph effective nerve repair in patients.
References
- Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. Apr 1997;62(4):310-318.
- Napoli I, Noon LA, Ribeiro S, et al. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. Feb 23 2012;73(4):729-742.
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. AnnuRevNeurosci. 2007;30:209-233.
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153-179.
- Arthur-Farraj PJ, Latouche M, Wilton DK, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. Aug 23 2012;75(4):633-647.
- Masaki T, Qu J, Cholewa-Waclaw J, Burr K, Raaum R, Rambukkana A. Reprogramming adult Schwann cells to stem cell-like cells by leprosy bacilli promotes dissemination of infection. Cell. Jan 17 2013;152(1-2):51-67.
- Zheng JQ, Kelly TK, Chang B, et al. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. Dec 1 2001;21(23):9291-9303.
- Court FA, Midha R, Cisterna BA, et al. Morphological evidence for a transport of ribosomes from Schwann cells to regenerating axons. Glia. Oct 2011;59(10):1529-1539.
- Nave KA. Myelination and the trophic support of long axons. Nature reviews Neuroscience. Apr 2010;11(4):275-283.
- Viader A, Golden JP, Baloh RH, Schmidt RE, Hunter DA, Milbrandt J. Schwann cell mitochondrial metabolism supports long-term axonal survival and peripheral nerve function. The Journal of neuroscience : the official journal of the Society for Neuroscience. Jul 13 2011;31(28):10128-10140.
- Kassmann CM, Quintes S, Rietdorf J, et al. A role for myelin-associated peroxisomes in maintaining paranodal loops and axonal integrity. FEBS letters. Jul 21 2011;585(14):2205-2211.
- Brown AM, Evans RD, Black J, Ransom BR. Schwann cell glycogen selectively supports myelinated axon function. Annals of neurology. Sep 2012;72(3):406-418.
- Fricker FR A-MA, Galino J, Paramsothy R, La Russa F, Perkins J, Goldberg R, Brelstaff J, Zhu N, McMahon SB, Orengo C, Garratt AN, Birchmeier C, and Bennett DLH. Axonal Neuregulin-1is a rate limiting but not essential factor for nerve remyelination. Brain : a journal of neurology. In Press 2013;In Press.
- Fricker FR, Lago N, Balarajah S, et al. Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience. Mar 2 2011;31(9):3225-3233.
- Stassart RM, Fledrich R, Velanac V, et al. A role for Schwann cell-derived neuregulin-1 in remyelination. Nature neuroscience. Jan 2013;16(1):48-54.
- Ozkaynak E, Abello G, Jaegle M, et al. Adam22 is a major neuronal receptor for Lgi4-mediated Schwann cell signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. Mar 10 2010;30(10):3857-3864.
- Maurel P, Einheber S, Galinska J, et al. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol. Aug 27 2007;178(5):861-874.
- Spiegel I, Adamsky K, Eshed Y, et al. A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nature neuroscience. Jul 2007;10(7):861-869.
- Zelano J, Plantman S, Hailer NP, Cullheim S. Altered expression of nectin-like adhesion molecules in the peripheral nerve after sciatic nerve transection. Neuroscience letters. Jan 2 2009;449(1):28-33.
- Monk KR, Oshima K, Jors S, Heller S, Talbot WS. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development. Jul 2011;138(13):2673-2680.
- Li H, Terenghi G, Hall SM. Effects of delayed re-innervation on the expression of c-erbB receptors by chronically denervated rat Schwann cells in vivo. Glia. Aug 1997;20(4):333-347.
- Hoke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. Jan 2002;173(1):77-85.
- Krajewski KM, Lewis RA, Fuerst DR, et al. Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1A. Brain : a journal of neurology. Jul 2000;123 ( Pt 7):1516-1527.
- Tapinos N, Ohnishi M, Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat Med. Aug 2006;12(8):961-966. 25. La Marca R, Cerri F, Horiuchi K, et al. TACE (ADAM17) inhibits Schwann cell myelination. Nature neuroscience. Jun 12 2011;14(7):857-865.

