Article being reviewed: Ohkawa T, Satake S, Yokoi N, Miyazaki Y, Ohshita T, Sobue G, Takashima H, Watanabe O, Fukata Y, Fukata M. Identification and characterization of GABAA receptor autoantibodies in autoimmune encephalitis. J Neurosci. 2014;34:8151-63.
Summary
- Antibodies against the β3 subunit of GABAA receptors identified in patients with thymomas.
- Spectrum of autoimmune encephalitides extended with discovery of pathogenic antibodies to inhibitory channel.
- Identification of antibodies involved a comprehensive characterisation of pathogenicity.
- Clinically, improvement is observed but coincides with multiple interventions and does not directly address whether this may be due to a depression in autoantibody titres.
- Binding of antibodies may alter network excitability, as inhibitory neurotransmission is likely to be impaired.
Over the past decades, our understanding of the interactions between the immune system and the brain has been challenged. Research showed that antibodies against central nervous structures can be produced by our own immune system often without an identifiable cause. This can lead to loss of the target antigen and inflammation of brain tissue. These autoantibody-mediated conditions are collectively referred to by the term autoimmune encephalitides. Patients typically present with subacute onset of memory loss, psychiatric disturbance, confusion, seizures, and in some cases abnormal movements. The targets of these pathogenic autoantibodies have been identified as receptors or ion channel-associated proteins expressed in the central nervous system (CNS) – the N-methyl-D-aspartate (NMDA) receptors and the voltage-gated potassium channel (VGKC) complex proteins are the most commonly identified autoantibody targets. Whilst initially considered a purely paraneoplastic phenomenon associated with tumours outside of the CNS1, autoantibodies against CNS antigens were shown to be present in patients without an underlying, or diagnosed, neoplasm2.
Whether the autoantibodies are pathogenic per se, or whether they are merely a marker coinciding with a separate disease process are questions that have been the focus of study. Antibodies mediate their pathogenicity in several ways but the most common mechanisms are internalisation of their antigenic target, activation of the lytic complement cascade or directly interference with ion channel function (discussed in Vincent et al. (2011)3 in more detail). Irrespective of their pathogenic mechanism, the first step always involves binding of the antibody to the antigen: an important paradigm, therefore, is that autoantibodies against cell surface receptors are more likely to be pathogenic than autoantibodies against intracellular antigenic targets.
To determine an autoantibody’s pathogenicity, Koch’s postulates on infectious diseases were modified to apply to autoimmune conditions4. Autoantibody-mediated pathogenicity can be assumed in cases where (1) an antibody-mediated immune response is present and (2) the antigen has been identified. Furthermore, the postulates require that the disease be induced experimentally, both in a (3) passive transfer and (4) an active immunisation model.
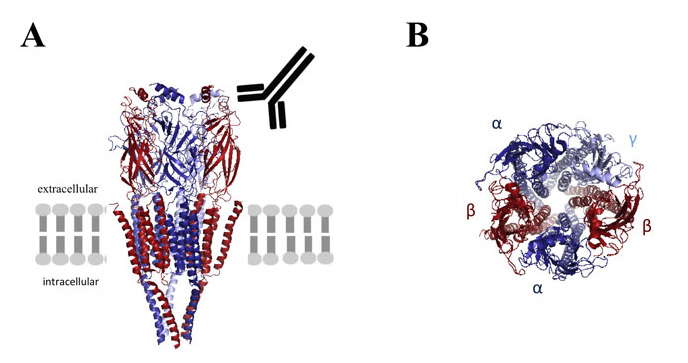
The three dimensional structure of the GABAA receptor is shown schematically in its position within the lipid bilayer (A) and its subunit composition is shown in more detail (B). Autoantibodies to the beta subunit (A) recognise the extracellular domain in vivo. *The programme database file of the related nicotinic acetylcholine receptor (2BG9) was used to model the GABAA receptor using MacPymol software.
Earlier this year, Petit-Pedrol et al (2014)5 identified autoantibodies to the γ-Aminobutyric acid (GABA)A receptors in patients with encephalitis who presented with intractable seizures or status epilepticus with no tumours. The antibodies were shown to bind to the α1 or β3 subunits. Antibodies to the GABAA receptors have also been found in a proportion of patients referred for NMDAR antibody testing (Pettingill et al, submitted). GABAA receptors are postsynaptic GABA-gated pentameric channels made up from 2α, 2β and 1γ subunits surrounding a central ion-selective chloride channel. Their main function is to depress neuronal excitability6,7.
The paper by Ohkawa et al (2014)8 identified novel autoantibodies to the β3 subunit of the GABAA receptor in two patients who presented with clinical manifestations of confusion, personality changes, memory loss, and seizures and examined in more detail the possible pathogenic mechanisms. Both patients had invasive cancers of their thymus, which required surgical excision and radiotherapy. They were identified from a cohort of over 100 patients with suspected autoimmune pathology of the CNS by screening patient sera binding to primary hippocampal cultures. The identity of the antigenic target was examined by using a combination of immunoprecipitation and mass spectrometry. Expression of individual GABAA demonstrated that the autoantibodies bound an extracellular epitope on the β3 subunit. The autoantibodies did not bind other GABAA receptor subunits, though evidence of cell surface expression of individual subunits was not provided. However, a β3-subunit-specific knockdown experiment confirmed that the autoantibodies no longer bound the hippocampal neuron surface when the β3 subunit was removed from the channel complex. The autoantibodies downregulated surface GABAA receptors over 48 hours in neuronal cultures, consistent with the internalisation mechanisms; the reduction of cell surface ion channels was not mediated by the complement pathway. Additionally, the reduction in cell surface GABAA receptor levels was also matched by a depression in electrophysiological activity. These autoantibody-mediated effects were specific to patient serum obtained during the manifestation of CNS symptoms; archived serum from one of the patients predating the encephalitis did not affect GABAA receptor numbers or electrophysiological recordings.
Clinically, the distinction between paraneoplastic and non-paraneoplastic autoantibodies may aid the treatment decision: a sustained immune response raised against the neoplasm can be limited by excision of the tumour, whereas non-paraneoplastic autoantibodies can only be targeted by immunosuppressive therapy. Steroids, plasma exchange and intravenous immunoglobulins are often used as first step immunosuppressants, and more aggressive treatment approaches have been used for resistant or relapsing patients9. No large studies have been performed to date to compare treatment strategies.
Clinical improvement of one of the patients was seen after administration of immunosuppressive therapy (corticosteroids and intravenous immunoglobulins) combined with anti-epileptic drugs. The patient became seizure-free, though cognitive and psychological symptoms persisted. Autoantibody levels were quantified using a cell-based enzyme-linked immunosorbent assay (ELISA) prior to immunotherapy only and it remains unclear whether a depression in autoantibody titres following therapy may have coincided with the alleviation of symptoms. Patient 2 was treated with chemotherapy alone, and whether the improvement was due to a treatment-related immunosuppression or a reduction in tumour load affecting (paraneoplastic) autoantibody levels remains also unclear. As both patients had invasive thymomas, a paraneoplastic phenomenon may have been likely. Therefore, histological analysis showing the potential expression of GABAA receptor subunit within the tumour tissue would have been useful. The use of a semi-quantitative approach to measure autoantibody levels with cell-surface ELISA or similar methods, would have also allowed the investigation of the temporal relationship between clinical status and autoantibody levels more closely.
The presence of VGKC-complex autoantibodies in both patient sera further complicates the conclusion as to whether anti-β3 GABAA receptor autoantibodies are specifically responsible for the clinical features. It is also possible that the full spectrum of anti-VGKC-complex associated antibodies has not been identified as yet. Screening of larger patient cohorts with similar CNS features might be helpful in future to address whether GABAA receptor autoantibodies are solely linked to invasive thymomas and whether the co-existence of VGKC-complex autoantibodies is typical for this patient group. This detailed characterisation of the GABAA receptor autoantibody emphasises the importance for the continued screening for novel CNS antigens in patients with encephalitis-like symptoms.
Antibody-mediated pathology was once thought to be rare but since the discovery of autoantibodies against the NMDA receptors1, at least thirteen types of autoimmune encephalitis have been described in a rapidly expanding clinical field. Pathogenic antibodies against subunits of inhibitory receptors described to date have included those against the GlyαR1 subunit of the glycine (Gly) receptors10, and those against the B1 subunit of the GABAB receptors11.
Pathogenic antibody binding to synaptic cell surface or structural proteins of inhibitory channels is likely to interfere with inhibitory neurotransmission in the CNS. This would be supported by the cessation of seizures, a possible surrogate of hyperexcitability, when immunotherapy suppresses autoantibody titres. A close study of correlation of autoantibody levels in serum and cerebrospinal fluid and their temporal relationship to symptoms is thus important.Autoantibodies have been linked with hyperexcitability in the case of the VGKC-complexes12,13, GABAB11, and Gly receptors10. 30-40% of neurons in the CNS use GABA as their neurotransmitter and inhibitory effects are predominantly mediated via the GABAA receptors. Okhawa et al (2014)8 demonstrated that autoantibody levels depressed inhibitory currents of surface GABAA receptors but did not have any effects on excitatory currents mediated by AMPA receptors. It is likely that a prolonged exposure (>24 hrs) to the antibodies may have altered excitatory neurotransmission as this could mimic better in vivo conditions. Local pathological inflammation may also contribute to excitability in vivo. A localised immune response with the subsequent release of cytokines and a possible element of complement activation might further impact onto local neuronal signalling pathways. Activated microglia and reactive astrocytes may alter the balance between excitation and inhibition in the milieu and may affect neuronal wiring through the formation of a glial scar.14
Blood-brain barrier integrity could be affected through cytokine-activated receptors on endothelial cells, leading to a further recruitment of immune cells to the CNS. Thus, the effects mediated by pathogenic antibodies against inhibitory channels calls for the need to develop comprehensive in vivo human studies and animal models to determine autoantibody-mediated pathogenicity on a molecular, network and more global level. Understanding how autoantibodies can cause specific symptoms would help us understand not only disease but also brain function2. Clinicians should be guided by the neuropsychiatric symptoms to identify whether an autoimmune cause should be ruled out mainly because the immunotherapy provides clinical improvements. The findings of Ohkawa et al (2014)8 extend the clinical spectrum of autoimmune encephalitides to include the GABAA receptors and strongly suggest that future research should focus on further screening of larger patient cohorts to elucidate the downstream effects of autoantibody binding to postsynaptic receptors.
References
- Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25-36.
- Irani SR, Gelfand JM, Al-Diwani A, Vincent A. Cell-surface central nervous system autoantibodies: Clinical relevance and emerging paradigms. Ann Neurol. 2014;76:168-84.
- Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol. 2011;10:759-72.
- Witebsky E. Experimental evidence for the role of autoimmunization in chronic thyroiditis. Proc R Soc Med. 1957;50: 955-8.
- Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, McCracken L, Martinez-Hernandez E, Mason WP, Kruer MC, Ritacco DG, Grisold W, Meaney BF, Alcalá C, Sillevis-Smitt P, Titulaer MJ, Balice-Gordon R, Graus F, Dalmau J. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276-86.
- Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem. 2012;287:40224-31.
- Miller PS, Aricescu AR. Crystal structure of a human GABAA receptor. Nature 2014;512:270-5.
- Ohkawa T, Satake S, Yokoi N, Miyazaki Y, Ohshita T, Sobue G, Takashima H, Watanabe O, Fukata Y, Fukata M. Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. J Neurosci. 2014;34:8151-63.
- Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study.Lancet Neurol. 2013;12:157-65.
- Carvajal-González A, Leite MI, Waters P, Woodhall M, Coutinho E, Balint B, Lang B, Pettingill P, Carr A, Sheerin UM, Press R, Lunn MP, Lim M, Maddison P, Meinck HM, Vandenberghe W, Vincent A. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain 2014; 137:2178-92.
- Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, Friedman D, Skeen MB, Grisold W, Kimura A, Ohta K, Iizuka T, Guzman M, Graus F, Moss SJ, Balice-Gordon R, Dalmau J. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010; 9:67-76.
- Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, Lang B, Bien CG, Vincent A. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010; 133: 1655-67.
- Lancaster E, Huijbers MG, Bar V, Boronat A, Wong A, Martinez-Hernandez E, Wilson C, Jacobs D, Lai M, Walker RW, Graus F, Bataller L, Illa I, Markx S, Strauss KA, Peles E, Scherer SS, Dalmau J. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia.Ann Neurol. 2011; 69:303-11.
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137-49.

