Summary
- The highly potent dopamine agonist apomorphine is the only drug whose antiparkinsonian efficacy equals that of levodopa.
- When given as single SC injection, it leads to motor improvement within minutes and provides the most rapid and reliable relief from OFF symptoms currently available.
- When administered as continuous SC infusion, apomorphine enables a reduction in oral drugs and, in keeping with the concept of continuous drug delivery, may lead to marked improvements in patients with motor complications that have become refractory to adaptations of oral and transdermal treatments.
- As the least invasive among the device-aided treatments, apomorphine should be considered in all patients with refractory motor fluctuations that have a negative impact on patients´ quality of life.
Dopaminergic replacement therapies typically improve motor problems in Parkinson´s disease (PD). Over the disease course, however, the response to levodopa doses becomes shorter and patients become aware of the recurrence of their parkinsonian symptoms at the end of the dose effect. The management of these motor fluctuations may be relatively straightforward while OFF periods are limited to end-of-dose effects and while gastrointestinal absorption remains reliable.1 However, OFF periods may become refractory, and they may be associated with highly unpleasant non-motor symptoms.2 Frequently, it is the emergence of involuntary movements associated with ON periods that makes management of motor fluctuations complex and further reduces quality of life.3 Although our understanding of the mechanisms underlying motor complications remains incomplete, the short half-life of oral antiparkinsonian drugs such as levodopa is involved. Oscillations in plasma and synaptic dopamine concentrations are believed to induce maladaptive changes in basal ganglia motor circuits.1,4 This concept has been supported by animal studies and findings in PD patients, where the longer-acting dopamine agonists as initial treatment (instead of levodopa) can delay the onset of motor complications,5 ( while motor complications can be ameliorated by switching from short-acting oral drugs to the continuous application of the same drugs.
Infusion therapies that provide stable drug delivery are available for patients whose motor complications have become refractory to all adjustments to the oral medication. Both levodopa and apomorphine infusion have fewer contraindications than deep brain stimulation and therefore represent an option for a broader spectrum of patients. While intrajejunal levodopa infusion requires the insertion of a tube through the abdominal wall, apomorphine is administered subcutaneously. It is thus the least invasive and the most easily reversible of the device-aided treatments.
Apomorphine
Apomorphine is a non-ergot dopamine agonist which stands out among the dopamine agonists in several ways: It is the only drug with an effect on parkinsonian motor signs equal to that of levodopa. Due to its low bioavailability, it must be administered parenterally, usually subcutaneously. When injected, the drug leads to the most rapid relief from parkinsonian motor problems currently achievable with any drug.
The substance has been known since the 19th century and was in use for psychiatric and veterinary applications but only infrequently for PD symptoms. As the long-term complications associated with levodopa were recognised, apomorphine was investigated further and was re-introduced into clinical use in the UK, by Professor Andrew Lees and his team at UCL. Starting from this long tradition in the UK, it is now also licensed in many other countries, both as intermittent injection therapy and as SC infusion. Alternative modes of delivery have been explored and a sublingual formulation is being investigated further.
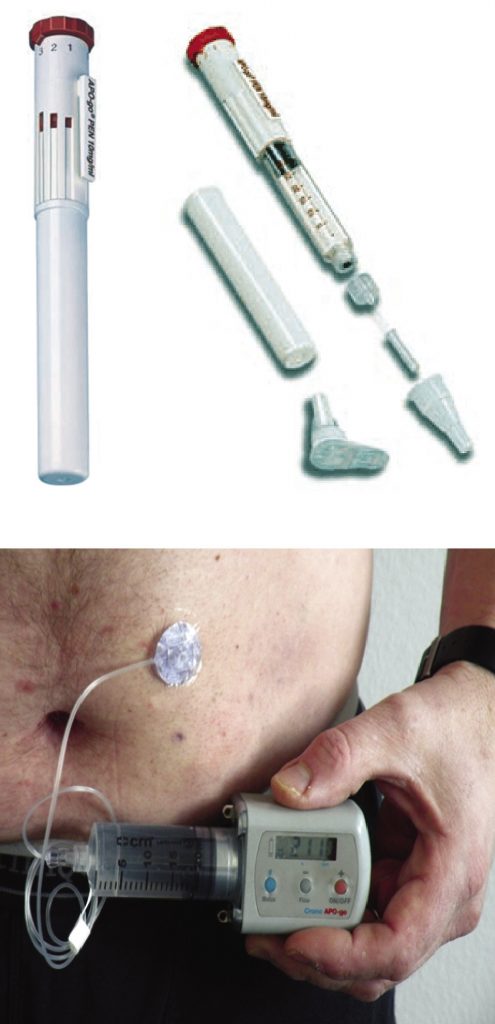
Injection therapy
Following a SC injection, the effect sets in after 5-20 (mean 7) minutes and lasts for around 40-60 minutes. Many open, uncontrolled studies of apomorphine injections as an add-on to oral treatment showed a mean reduction of daily OFF time by around 50% compared to baseline.6
Several randomised, placebo-controlled studies confirmed this effect.7,8 Importantly, using this treatment, motor improvement can be achieved in a very reliable manner, once each patient’s individually required dose has been established: A randomised study demonstrated that 95% of OFF periods were terminated by apomorphine, compared to 23% on placebo. As expected with a short-acting drug, ON time with troublesome dyskinesia also increased, although not significantly.7
This treatment is suitable for patients with refractory or troublesome OFF periods which persist despite optimised oral and transdermal treatment. Patients must be able to distinguish between OFF symptoms and other problems such as dyskinesia, and must be able to handle the injection device during an OFF phase; or must have a carer able to do so. The injections are usually administered using a pen, with the individually optimised dose pre-set.
Patients with troublesome dyskinesia during ON periods are less good candidates and all patients should be observed with regards to worsening or new onset of dykinesia.
Practical approach
To determine the individual dose, patients undergo a challenge test. This can be done on an out-patient basis, if required. Domperidone, a peripheral dopamine receptor blocker, is used for one to three days before starting apomorphine to avoid nausea. Domperidone has been linked to QT prolongations and in 2014, the European Medicines Agency limited the daily dose to 30mg and stated that the drug should not normally be used for longer than one week.9 Alternatively, trimethobenzamide may be used. A study of ondansetrone (without placebo arm) showed less efficacy than domperidone, albeit in untreated patients.10
Starting during an OFF period, increasing doses of apomorphine are injected until a full ON is achieved (or until intolerable, adverse side effects occur) and the patient is observed for motor response as well as tolerability. The first dose is usually 1 or 1.5mg, with increments of 1 or 1.5mg every 30-45 minutes, but more rapid titration schemes are also used.11
Continuous infusion therapy
Several uncontrolled studies showed marked reductions in daily OFF time from baseline when apomorphine is administered via continuous subcutaneous infusion during waking hours. The largest, retrospective, study was multi-centre and reported a reduction in daily OFF time by 4.3 hours.6 Randomised comparisons with other treatments have not been performed but a European placebo-controlled multi-centre study is on-going (Toledo Study).
Some uncontrolled studies also reported reductions in dyskinesia severity, by 34% up to 83%. One study using levodopa and apomorphine challenge tests before and six months after initiating apomorphine infusion showed a reduction in dyskinesia severity by 34-44% on blinded video ratings.12 Maximum dyskinesia improvement has been observed after several months.13 Dyskinesia reduction may be more marked in patients who manage to substantially reduce their oral therapy, and consequently, the mean daily apomorphine doses in studies reporting effects on dyskinesia have been in the range of 100 mg.13 “Apomorphine monotherapy” has been defined as infusion only during the waking day with discontinuation of oral drugs, except in the morning and at night.12,13,14 The determinant factor for dyskinesia reduction is likely the overall reduction of short-acting agents,1 and strict monotherapy is not necessarily required, particularly when the problem is refractory OFFs rather than dyskinesia. The observed improvement in motor complications is in keeping with the current concept and believed to be due to the replacement of pulsatile with continuous drug delivery.
There is some evidence suggesting relevant improvements in non-motor problems. A non-randomised study found a significant improvement in the overall score of the Non Motor Symptom Assessment Scale for PD (NMSS), from 106 (SD 65) to 56 (45) points (p=0.0003), including in sleep, mood, perception, attention, urinary and gastrointestinal symptoms, and this was associated with a significant improvement in quality of life.15
Practical approach
In practical terms, infusion treatment is usually initiated on an in-patient basis although this is not an absolute requirement if frequent visits are possible and increases in the flow rate are done slowly.16 An apomorphine challenge test is not required although some centres perform this to determine an approximate dose range that the patient will require. Tolerability cannot be judged sufficiently from a challenge test as slow increases in the hourly flow rate of the pump help to avoid adverse effects.
The pump is usually worn on a belt around the patient’s waist and the needle is inserted into the abdominal skin into rotating injection sites. During the initial in-patient stay, patients and carers are instructed in handling the pump, including hygiene measures. Oral dopamine agonists are usually withdrawn completely during the initial in-patient stay, and subsequently other oral antiparkinsonian drugs are gradually reduced or withdrawn over weeks and sometimes a few months while the flow rate of apomorphine is increased. While the standard daily duration of infusion is around 14-18 hours, some patients with severe nocturnal OFFs benefit from 24-hour administration, with lower doses at night.
Safety
Potential adverse effects of apomorphine include dopaminergic effects including nausea, orthostatic hypotension, leg oedema, or somnolence.
Skin nodule formation is very common on infusion – although usually mild to moderate – but rarely problematic with injection therapy. Rarely, abscesses or ulcerations occur on infusion therapy. Widespread nodules may impair reliable and stable absorption of apomorphine. Local treatments include massages, application of corticoid creams, or silicone patches. Only therapeutic ultrasound has been investigated in a randomised study, with results suggesting efficacy. 17
Haemolytic anaemia is rare (below 1%)6 but regular screening is required. Coombs Test has been described to turn positive in 6-12.5% although this may be reversible. Haemolytic anaemia requires discontinuation of apomorphine and treatment in collaboration with haematology specialists.
Neuropsychiatric adverse effects may occur. As with other dopaminergic drugs, vulnerable patients may develop impulse control disorders but no comparative studies exist to show whether these are more common than with other dopamine agonists. Other neuropsychiatric problems are typically associated with long disease duration. These include punding, a behavioural disorder with repetitive, prolonged activities resembling normal recreational or domestic activities (e.g. cleaning, using a computer); and dopamine dysregulation syndrome, a drug dependency syndrome with craving for increasing dopaminergic doses despite detrimental behavioural changes and often dyskinesia, which also occurs on high doses of levodopa. It is unknown whether confusion or hallucinations are more common than with oral dopamine agonists but there is evidence suggesting relatively good neuropsychiatric tolerability of apomorphine: A small non-randomised study showed improvements in the cognitive / emotional categories of the NMSS over one year in the apomorphine group but not in the medically treated group.15 Another non-randomised study found significant improvements in these categories in 43 patients on apomorphine infusion, with similar findings in patients on intrajejunal levodopa.18 Several small studies in patients using apomorphine infusion while on waiting lists for DBS showed stable scores on the Neuropsychiatric Inventory over several years.19 An uncontrolled multi-centre four-year study reported drop-outs due to neuropsychiatric adverse events in only 8 out of 166 patients.6
Technical problems rarely result in treatment discontinuation. Issues occasionally seen include clotting of connections, arrest of the pump, and disconnection of the syringe. Patients receive instructions on which oral / transdermal medication to use in case a technical problem cannot be dealt with immediately. Teams including a nurse with special interest in PD are best suited for the management of patients using infusion therapies for the motor complications of PD.
Choice of advanced PD treatment
The indication for SC apomorphine infusion is the same as for intrajejunal levodopa infusion and deep brain stimulation (DBS): motor complications which have become refractory to all adaptations of oral and transdermal treatments and which have a relevant impact on a patient’s quality of life.
It is usually advisable to discuss device-aided treatments early when a patient’s motor complications become difficult to manage, to reassure them that further options are available.20 The choice of treatment is ultimately the patients’ and the clinician must tell the patients and any caregivers which treatments are options for them (and why others are not). With all device-aided treatments, it is important to point out that the effect that can realistically be achieved is similar to each patient’s individual best ON state but ideally with less dyskinesia and fewer medication-induced adverse effects. Therefore, many problems that persist during ON will likely not be resolved by infusion therapies. Examples include balance problems, freezing, dysarthria and dysphagia during ON.
Contraindications for infusion treatments are less strict than for DBS. The spectrum of patients who are potential candicates for infusion therapies is wider than for DBS, particularly because there is no age limit and because infusions may be tried in patients with mild cognitive impairment or mild dementia if adequate caregiver support is available. Similarly, balance problems and falls during ON are important contraindications for DBS but not for the infusions, although they are unlikely to improve.
Due to the lack of randomised studies comparing levodopa and apomorphine infusion, it is not known whether there is a difference in their efficacy on motor fluctuations and dyskinesia. Their adverse effect profiles and contraindications differ somewhat, however: Levodopa infusion may be complicated by local infections including peritonitis and by technical complications, and has been linked to an increased risk of polyneuropathy. In contrast, adverse effects typically associated with dopamine agonists are likely more common with apomorphine, such as nausea, daytime sleepiness, orthostatic dysregulation, oedema and neuropsychiatric changes. If these problems pre-exist in a patient, they are relative or absolute contraindications for apomorphine, depending on the severity. Marked dementia is a contraindication for apomorphine but not necessarily for levodopa infusion, provided adequate care-giver support exists. However, apomorphine has the advantage of being less invasive and more easily reversible than levodopa infusion, allowing a lower threshold for a treatment trial.
As such, apomorphine infusion should be considered in patients as soon as motor fluctuations are becoming difficult to manage and it is likely that many more patients could benefit from apomorphine if this option were considered for all potential patients.
References
- Jenner P. Wearing off, dyskinesia, and the use of continuous drug delivery in Parkinson’s disease. Neurol Clin 2013;31(3 Suppl):S17-35.
- Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, Poncet M, Chérif AA. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology 2002;59:408-13.
- Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord 2015;30:80-9.
- Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A; LCIG Horizon Study Group. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 2014;13:141-9.
- Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M, Poewe W, Rascol O, Goetz CG, Sampaio C. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson’s disease. Mov Disord 2011;26 Suppl 3:S2-41.
- García Ruiz PJ, Sesar Ignacio A, Ares Pensado B, Castro García A, Alonso Frech F, Alvarez López M, Arbelo González J, Baiges Octavio J, Burguera Hernández JA, Calopa Garriga M, Campos Blanco D, Castaño García B, Carballo Cordero M, Chacón Peña J, Espino Ibáñez A, Gorospe Onisalde A, Giménez-Roldán S, Granés Ibáñez P, Hernández Vara J, Ibáñez Alonso R, Jiménez Jiménez FJ, Krupinski J, Kulisevsky Bojarsky J, Legarda Ramírez I, Lezcano García E, Martínez-Castrillo JC, Mateo González D, Miquel Rodríguez F, Mir P, Muñoz Fargas E, Obeso Inchausti J, Olivares Romero J, Olivé Plana J, Otermin Vallejo P, Pascual Sedano B, Pérez de Colosía Rama V, Pérez López-Fraile I, Planas Comes A, Puente Periz V, Rodríguez Oroz MC, Sevillano García D, Solís Pérez P, Suárez Muñoz J, Vaamonde Gamo J, Valero Merino C, Valldeoriola Serra F, Velázquez Pérez JM, Yáñez Baña R, Zamarbide Capdepon I. Efficacy of long-term continuous subcutaneous apomorphine infusion in advanced Parkinson’s disease with motor fluctuations: a multicenter study. Mov Disord 2008;23(8):1130-6.
- Dewey RB Jr, Hutton JT, LeWitt PA, Factor SA. A randomized, double-blind, placebo-controlled trial of subcutaneously injected apomorphine for parkinsonian off-state events. Arch Neurol 2001;58:1385-92.
- Pahwa R, Koller WC, Trosch RM, Sherry JH; APO303 Study Investigators. Subcutaneous apomorphine in patients with advanced Parkinson’s disease: a dose-escalation study with randomized, double-blind, placebo-controlled crossover evaluation of a single dose. J Neurol Sci 2007;258:137-43.
- http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Domperidone-containing_medicines/human_referral_prac_000021.jsp&mid=WC0b01ac05805c516f, accessed February 27, 2015.
- Arnold G, Schwarz J, Macher C, Oertel WH. Domperidone is superior to ondansetron in acute apomorphine challenge in previously untreated parkinsonian patients – A double blind study. Parkinsonism Relat Disord 1997;3:191-3.
- http://www.movementdisorders.org/MDS/Education/Latest-E-Learning/Device-Aided-Medical-Therapies.htm, accessed August 18, 2015.
- Katzenschlager R, Hughes A, Evans A, Manson AJ, Hoffman M, Swinn L, Watt H, Bhatia K, Quinn N, Lees AJ. Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson’s disease: a prospective study using single-dose challenges. Mov Disord 2005;20:151-7.
- Manson AJ, Turner K, Lees AJ. Apomorphine Monotherapy in the treatment of refractory motor complications of Parkinson’s Disease – A long-term follow-up study of 64 patients. Mov Disord 2002;17:1235-41.
- Colzi A, Turner K, Lees AJ. Continuous subcutaneous waking day apomorphine in the long term treatment of levodopa induced interdose dyskinesias in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1998;64:573-6.
- Martinez-Martin P, Reddy P, Antonini A, Henriksen T, Katzenschlager R, Odin P, Todorova A, Naidu Y, Tluk S, Chandiramani C, Martin A, Chaudhuri KR. Chronic subcutaneous infusion therapy with apomorphine in advanced Parkinson’s disease compared to conventional therapy: A real life study of non motor effect. J Park Dis 2011;1:197-203.
- Trenkwalder C, Chaudhuri KR, García Ruiz PJ, LeWitt P, Katzenschlager R, Sixel-Döring F, Henriksen T, Sesar Á, Poewe W. Expert Consensus Group report on the use of apomorphine in the treatment of Parkinson’s disease – Clinical practice recommendations. Parkinsonism Relat Disord 2015;21:1023-30.
- Poltawski L, Edwards H, Todd A, Watson T, Lees A, James CA. Ultrasound treatment of cutaneous side-effects of infused apomorphine: a randomized controlled pilot study. Mov Disord 2009;24:115-18.
- Martinez-Martin P, Reddy P, Katzenschlager R, Antonini A, Todorova A, Odin P, Henriksen T, Martin A, Calandrella D, Rizos A, Bryndum N, Glad A, Dafsari HS, Timmermann L, Ebersbach G, Kramberger MG, Samuel M, Wenzel K, Tomantschger V, Storch A, Reichmann H, Pirtosek Z, Trost M, Svenningsson P, Palhagen S, Volkmann J, Chaudhuri KR. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord 2015;30:510-16.
- Morgante L, Basile G, Epifanio A, Spina E, Antonini A, Stocchi F, Di Rosa E, Martino G, Marconi R, La Spina P, Nicita-Mauro V, Di Rosa AE. Continuous apomorphine infusion (CAI) and neuropsychiatric disorders in patients with advanced Parkinson’s disease: a follow-up of two years. Arch Gerontol Geriatr Suppl 2004;9:291-6.
- Odin P, Chaudhuri RK, Slevin JT, Volkmann J, Dietrichs E, Martinez-Martin P, Krauss JK, Henriksen T, Katzenschlager R, Antonini A, Rascol O, Poewe W. Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: consensus from an international survey and discussion program. Parkinsonism Relat Disord 2015;21:1133-44.
