Summary
- There is a major need for novel therapies in depression. One that has been suggested is transcranial direct current stimulation (tDCS), a form of mild, non-invasive electrical brain stimulation
- tDCS has been used effectively as an intervention for major depression, both alone and as an augmentative therapy combined with antidepressant medication
- Few studies have explored the neural mechanisms underlying the putative antidepressant effect of tDCS
- An improved understanding of the mechanisms underlying the antidepressant effect of tDCS is vital for enhancing clinical efficacy and predicting response to treatment, but this technique shows great potential as a safe, effective, and cost-effective therapy
The leading global cause of disability is major depression, affecting over 350 million people worldwide.1 Pharmacological and psychological therapies for depression have changed very little in the past thirty years, despite extensive research for new treatment targets. The most common antidepressant drugs are effective for about 58% of depressed patients in primary care, while 45% respond to placebo.2 The difficulty in developing new treatments arises in part because depression is a disorder of unknown aetiology. The neurobiological correlates of depression, on the other hand, are not entirely unknown: neuroscience research has identified several brain circuits that operate abnormally in depression. Recently, this knowledge has contributed to the development of experimental treatments, including those that stimulate the brain directly in a targeted manner. Among these novel treatments is a form of painless, noninvasive brain stimulation termed transcranial direct current stimulation (tDCS), commonly applied to the dorsolateral prefrontal cortex (DLPFC) in trials for depression. The practical advantages of tDCS are many: tDCS is comparatively inexpensive, portable, and safe. In this article we discuss the evidence that tDCS is effective in depression, and the neural and cognitive mechanisms that may drive its putative antidepressant effect. We also outline the importance of mechanistic studies of DLPFC tDCS to clarify its effects on the brain, and optimise its potential for clinical use.
What does tDCS do?
The use of weak electrical currents to stimulate the brain is not new, with reports dating back to the early 19th century.3 Mild brain stimulation such as tDCS has benefited from recent computational modelling and neuroimaging studies aimed at refining stimulation parameters, including electrode size, current amplitudes, location and durations of stimulation.4 tDCS uses a weak electric current to stimulate a localised area of the brain (‘direct’ indicates that the current flows in a single direction, in contrast to ‘alternating’ current stimulation). In early literature it was simply termed “polarisation”, referring to its effects on neuronal resting potentials.5
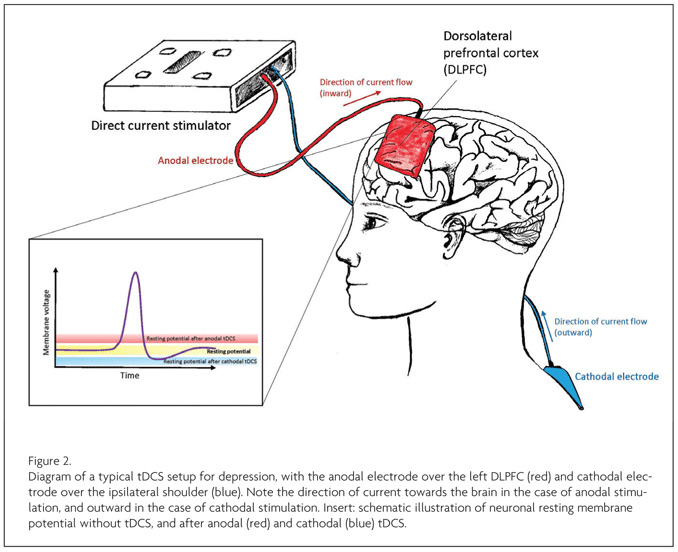
Recent depression trials place the anodal electrode over the left DLPFC, with the cathodal electrode used as a ‘ground’ electrode, for instance supraorbitally. In this specific example, this creates an electrical circuit with current travelling from the anodal (positive) to the cathodal (negative) electrode. This delivers low levels of electrical stimulation through the skull immediately under the anode, typically between 1 and 2 milliamps, with a small proportion of that affecting the cortex beneath, facilitating (but not directly evoking) neuronal activity in part through its effects on the resting potential (see below). Although the entire physiological effect is likely more complex, this general potentiation of activity has been reported to produce marked behavioural effects in domains as varied as motor tasks, auditory learning, and language.6 It is because of different electrode montages—electrode placement and current amplitude—that this technology can claim to modulate such an improbably diverse set of cognitive processes, including those involved in depression.
Why target the DLPFC?
One of the most established neural correlates of depression is abnormal metabolism in the DLPFC. Resting-state studies (e.g. using positron emission tomography: PET) tend to find a reduction in DLPFC activity in depressed patients, which normalises somewhat following symptom remission; by contrast, task-related activation (e.g. during working memory) is generally increased in the DLPFC in depression.7 Lesion studies also support the hypothesis of DLPFC dysfunction in depression: patients with lesions involving the DLPFC show higher levels of depression than patients with lesions sparing the DLPFC.7
Increased task-related DLPFC neural activation is often interpreted as cortical inefficiency. While the primary role of the DLPFC is in so-called ‘executive’ function and cognitive control, it also plays a central role in emotion regulation, particularly in the reappraisal and suppression of negative emotions.7 Thus, targeting the DLPFC with tDCS aims to remedy inefficiency in emotion regulation that could be central to depressive symptoms.
Clinical trials of tDCS in depression
The first double-blind, sham-controlled clinical trial of tDCS for depression reported very pronounced effects of tDCS on mood symptoms8 following a relatively short stimulation period. Differences between active and sham (placebo) tDCS even persisted over the succeeding month.
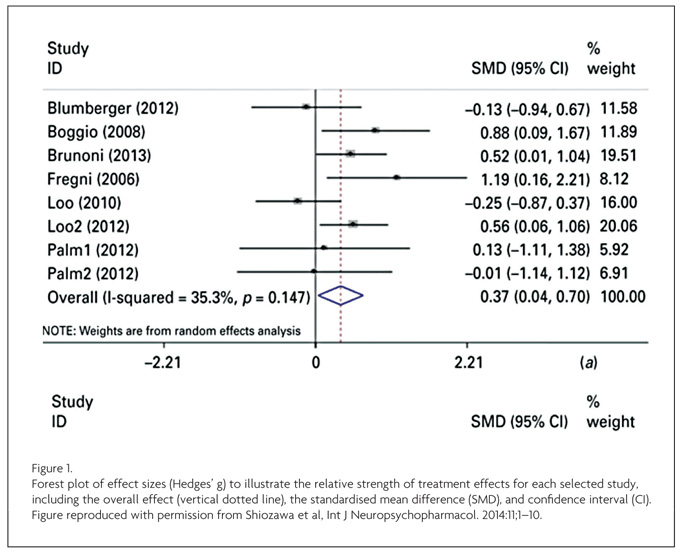
Less marked (but nonetheless substantial) effects of tDCS were later reported in a three-week trial: tDCS improved mood significantly more than sham, but no difference between active and sham was found in response rates.9 Cognitive effects were also smaller than reported in previous trials, with attention and working memory improvements found after the first tDCS session, but no (expected) cumulative cognitive enhancements independent of mood effects. More recently, a clinical trial found tDCS and sertraline to have comparable effects, but combined tDCS-sertraline treatment was more effective than either treatment alone.10 However, null results have also been reported, and some studies find substantially larger effect sizes than others (see Figure 1).11
Differences in stimulation parameters, outcome measures, and timing of tDCS delivery could all account for variation in tDCS effectiveness. In the next few years, clinical trials should clarify the optimal schedule for tDCS intervention in depression (typically trials have administered stimulation five times per week), and only after this can large-scale trials determine whether it should be offered as an alternative treatment option. Very few serious side effects have been reported in any tDCS trials for depression, with the most common including temporary itching, tingling, or skin redness, and very limited reports of hypomania.9,10 Thus, tDCS has great potential as a novel, safe, effective treatment for depression, but this can be realised only if the specific mechanisms and optimal stimulation parameters can be identified.4
Discovering the mechanisms of tDCS in depression
Advances in the clinical development of tDCS will be boosted by improved understanding of the neurophysiological mechanisms underlying its behavioural effects. tDCS has polarity-specific effects on neuronal membrane potential: anodal tDCS decreases the resting membrane potential of the neuronal soma, increasing the likelihood of depolarisation, whereas cathodal tDCS raises the membrane potential of the soma towards hyperpolarisation12 (see Figure 2). More widespread neural changes (i.e. away from the stimulation site), and local non-neuronal effects have also been reported, including local changes in ionic concentrations, levels of cyclic adenosine monophosphate (cAMP), protein synthesis, and NMDA receptor efficacy.4,6
Unfortunately for the purposes of understanding the effect of tDCS in depression, the neurophysiological mechanisms of tDCS have been studied predominantly in the motor cortex. The mechanisms driving ‘antidepressant’ tDCS over the DLPFC are poorly understood, though most studies corroborate the simple anodal-excitatory/cathodal-inhibitory model (though note that mild anodal currents can sometimes inhibit neuronal activity13).
One study investigating the neurophysiology of DLPFC tDCS found a polarity-specific effect on both working memory performance and EEG measures: anodal tDCS increased both behavioural performance and its electrophysiological correlates, while cathodal stimulation decreased both measures.6 In currently-depressed patients, tDCS was reported to induce neurophysiological changes extending over the medial frontal cortex during working memory performance.14 These studies provide insight into the role of the DLPFC in depression, indicating that tDCS might alter DLPFC efficiency as well as its interaction with more widespread prefrontal regions, driving the resulting behavioural and mood changes.
To date, most research on tDCS for depression has adopted one of two strategies: trials to determine whether tDCS improves depressive symptoms; or experimental studies, usually in healthy subjects, to determine its mechanism of effect. This division inhibits a better understanding of the specific mechanisms driving the antidepressant effects of tDCS. Studies combining both strategies have the ability to resolve this, and determine how tDCS could best target symptoms of depression.
Proceed cautiously – but not too cautiously
The most well-known electrical therapy in psychiatry is electroconvulsive therapy (ECT). Although evidence strongly indicates that ECT is an effective short-term treatment for depression,15 it is perceived negatively by the public and its therapeutic efficacy is often tempered by concerns about cognitive side-effects. In the case of tDCS, which uses stimulation currents that are orders of magnitude lower than ECT, initial reports of cognitive enhancement with tDCS reignited public interest in electrical current stimulation. From these reports emerged an online community of ‘home kit’ tDCS users who report finding amazing effects, sometimes far beyond what has been shown experimentally. Within the neuroscience community, researchers have begun to question the magnitude of the reported benefits of tDCS.16 An improved understanding of its mechanisms and effects is key to resolving these debates.
The challenge for basic and translational tDCS researchers is to convince clinicians that it is a credible experimental treatment, with the caveat that understanding its mechanisms will no doubt involve many more years of cellular, neurophysiological, and cognitive research. Putting treatment before mechanism is common in psychiatry: the neural mechanisms of proven effective treatments—cognitive therapy, electroconvulsive therapy, and many psychiatric drugs—are still relatively poorly understood. Still, history need not dictate future treatment discovery, and the tools of neuroscience can illuminate the biological and behavioural effects of tDCS alongside tests of its clinical efficacy. tDCS is only one of a number of experimental treatments for depression with origins in neuroscience, but it may hold particular potential as a new therapy, or as an augmentative strategy for existing therapies. The key will be honing the technology to target the complex and heterogenous nature of this debilitating disorder.
References
- World Health Organization. Depression. 2012.
- Arroll B, et al. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: a meta-analysis. Ann. Fam. Med. 2005;3:449-56.
- Fritsch G, Hitzig E. Neurosurgical Classics. Thieme Medical Publishers, Inc. 1992.
- Arul-Anandam AP, Loo C. Transcranial direct current stimulation: a new tool for the treatment of depression? J. Affect. Disord. 2009;117:137-45.
- Bishop G, O’Leary J. The effects of polarizing currents on cell potentials and their significance in the interpretation of central nervous system activity. Electroencephalogr. Clin. Neurophysiol. 1950;2:401-16.
- Zaehle T, Sandmann P, Thorne JD, Jäncke L, Herrmann CS. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: combined behavioural and electrophysiological evidence. BMC Neurosci. 2011:12:2.
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009;201:239-43.
- Fregni F et al. Treatment of major depression with transcranial direct current stimulation. Bipolar Disord. 2006;8:203-4.
- Loo CK et al. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br. J. Psychiatry 2012;200:52-9.
- Brunoni AR et al. The sertraline vs electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 2013;70:383-91.
- Shiozawa P et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 2014;1-10.
- Wachter D et al. Transcranial direct current stimulation induces polarity-specific changes of cortical blood perfusion in the rat. Exp. Neurol. 2011;227:322-7.
- Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport 1998;9:2257-60.
- Powell TY, Boonstra TW, Martin DM, Loo CK, Breakspear M. Modulation of Cortical Activity by Transcranial Direct Current Stimulation in Patients with Affective Disorder. PloS One 2014;9:e98503.
- The U. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. The Lancet 2003;361:799-808.
- Horvath JC, Forte JD, Carter O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations from Single-Session Transcranial Direct Current Stimulation (tDCS). Brain Stimulat. 2015.

