Abstract
There is class I evidence to support the use of surgery in the management of medically refractory epilepsy,1 with better clinical outcomes and cost effectiveness. Despite this, surgery remains an underutilised resource. One reason is that epilepsy is a complex and heterogeneous condition, and individuals require comprehensive pre-operative evaluation to determine whether surgery is appropriate for them. Surgery may be curative, with the aims of achieving seizure freedom, or palliative, with the aim of reducing seizure frequency. Surgery may also be diagnostic, with intracranial EEG playing an important role in the presurgical evaluation. In this article, the process of presurgical evaluation is summarised, followed by an overview of current surgical treatments available.
Presurgical Evaluation
Cortical zones
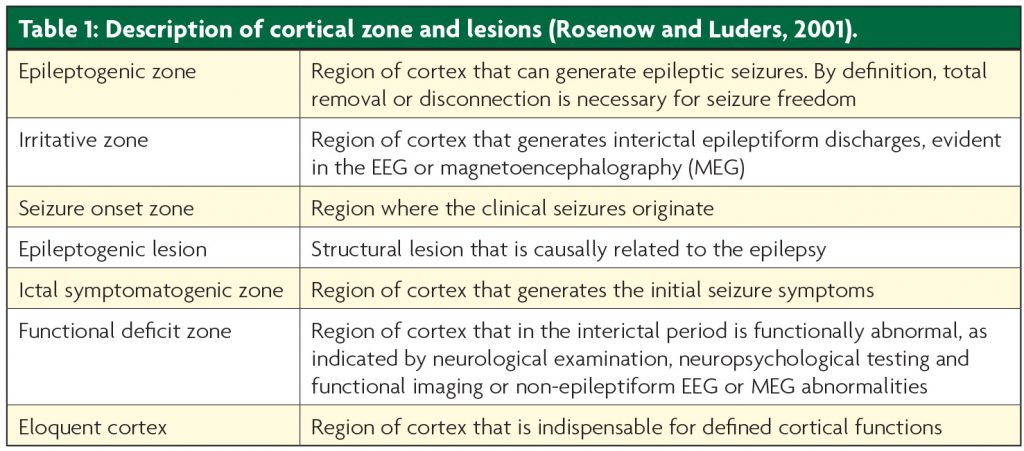
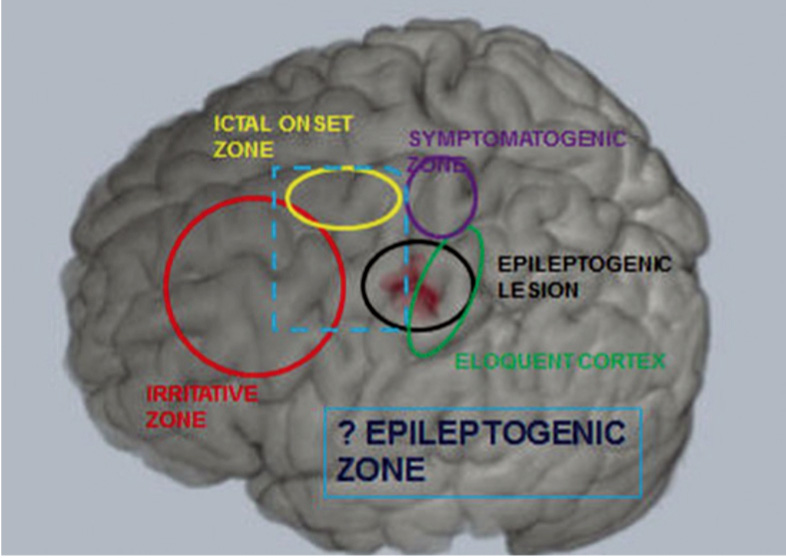
Six cortical zones have been defined in the presurgical evaluation of patients for epilepsy surgery2 (see Table 1, Figure 1). The epileptogenic zone (EZ) is defined as the area of cortex indispensible for the generation of clinical seizures. There is no single diagnostic test for the EZ, and it can only be identified retrospectively, with long-term seizure freedom following cortical resection. The aim of presurgical evaluation is to infer the localisation of the EZ, and ensure that this can be safely resected without causing significant deficits.
Patient selection
There are four general criteria necessary for patients to meet to be considered candidates for presurgical evaluation and resective surgery.
- Drug resistant epilepsy
- Clinical diagnosis of focal seizures
- Absence of contra-indications for presurgical evaluation and epilepsy surgery
- Declaration by the informed patient and/or carer that he/she wishes to undergo presurgical evaluation
General pathway for presurgical evaluation
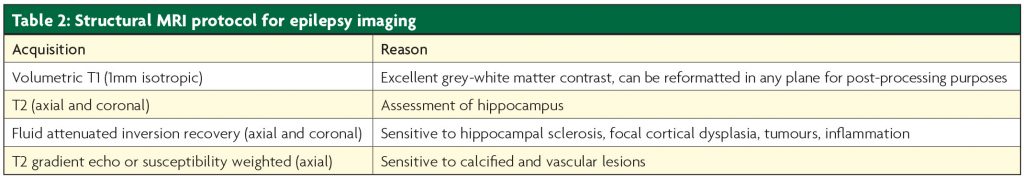
The general pathway followed by most Epilepsy Surgery Units is described in Figure 2.3 The initial clinical evaluation and clinical investigations are commonly referred to as Phase 1 in the process,4 and are composed of clinical evaluation of seizure semiology, scalp EEG and video telemetry, structural imaging (MRI) and neuropsychological and psychiatric assessment. See Table 2.
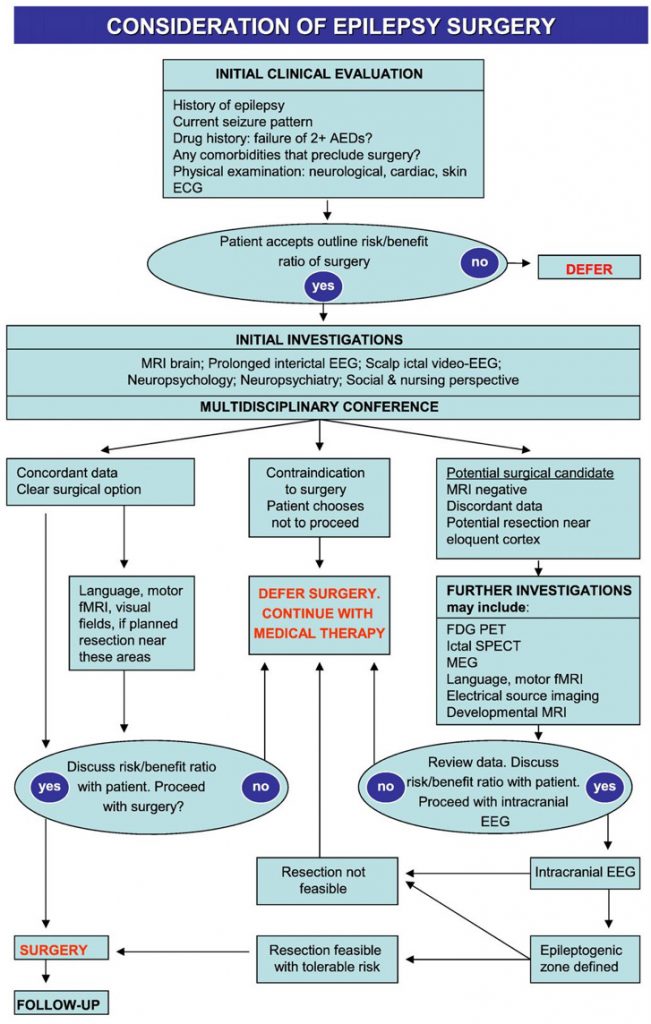
If the outcome of Phase 1 is a clear hypothesis for the site of the EZ, in an area that is surgically accessible with concordant investigations, then the recommendation may be to proceed with resective surgery. If there is uncertainty on the localisation of the EZ, further investigations are often necessary. This is known as Phase 1.5, and includes advanced imaging techniques and intracranial EEG (see Table 3).
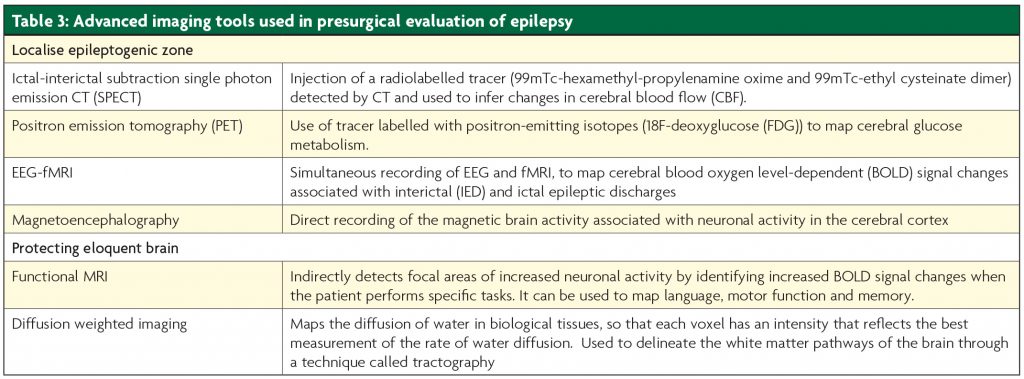
There is great benefit in integrating all available imaging data on 3D multimodality brain reconstructions to guide decision making and planning of surgery5 (Figure 3). 3D multi- modality image integration is especially useful in more complex patients with extratemporal epilepsy who undergo advanced imaging and intracranial EEG implantation.
Surgery
Intracranial EEG
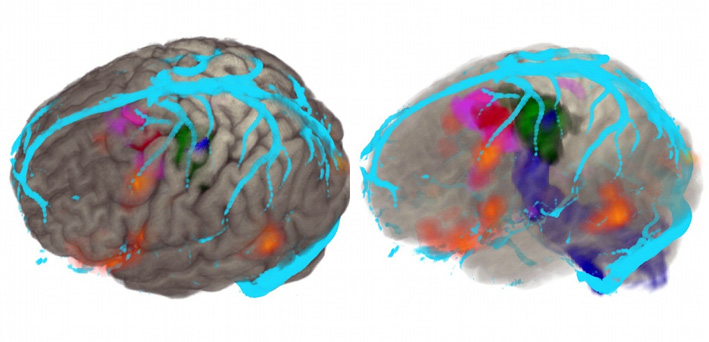
Intracranial EEG monitoring (ic-EEG) is indicated in patients with medically intractable focal epilepsy, where non-invasive investigations have failed to FInd a focus.6 Ic-EEG remains the gold standard for identifying the region of tissue that must be removed to ameliorate seizure activity. The decision to proceed to ic-EEG, and the precise location and configuration for surgery, arises from a multi-disciplinary case review with all the non-invasive investigations. Subdural grid electrodes are used to capture foci at the cortical surface (Figure 4). Depth electrodes placed percutaneously are used to capture activity in the deep cortical and subcortical structures, including the hippocampus, amygdala, insula, cingulate gyrus and areas of cortical dysplasia at the depth of a sulcus. The capture of foci in a three dimensional way is best achieved with stereoelectroencephalography (SEEG) (Figure 5).
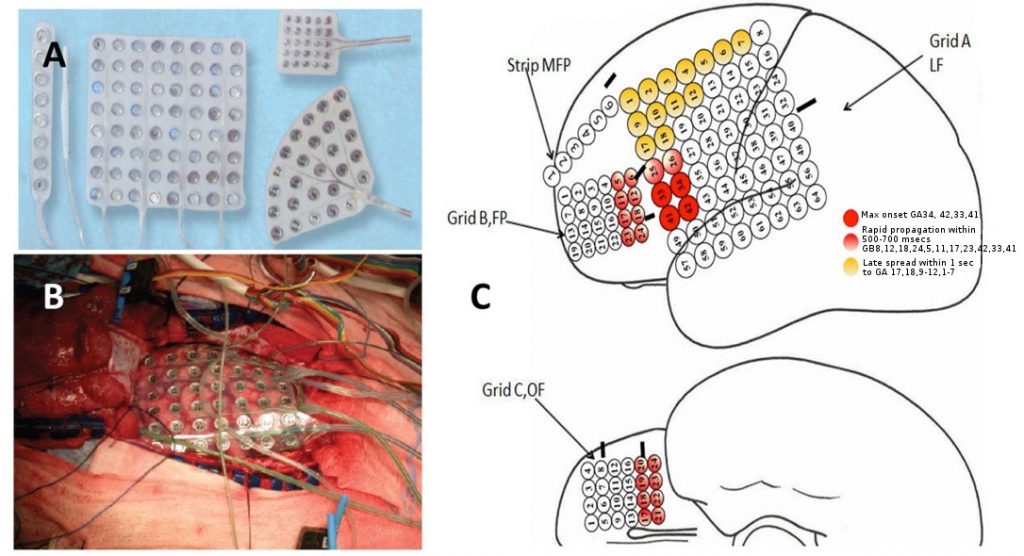
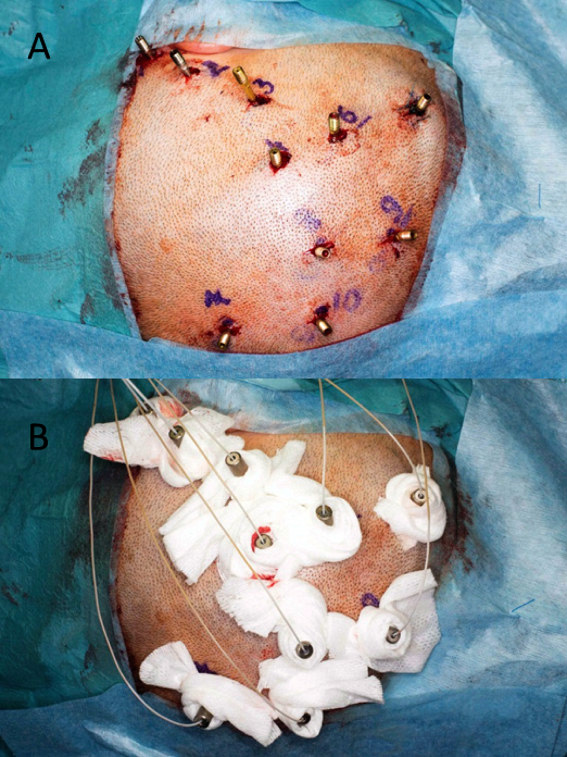
SEEG requires precise imaging of the intracranial arteries and veins to avoid vascular injury during insertion, a planning station to design electrode arrangements and a robust method to accurately execute the trajectories. The most accurate techniques use frame-based stereotactic techniques (Leksell, Brown-Roberts-Wells, Cosman-Roberts-Wells systems) and robot implementation,7-9 although there is increasing interest in frameless stereotactic techniques using custom-designed guidance tools.10
The ic-EEG implantation strategy should be tailored for individual patients, following discussion between the neurophysiologist and the neurosurgeon at the Multi-disciplinary telemetry meeting. Patients with seizure onset at the cortical surface, close to eloquent areas, will be more suited to subdural grid implantation, whereas patients with seizure onset at the depths of a sulcus, or inaccessible areas of cortex, are more likely to benefit from SEEG.11 Historically epilepsy surgery units tended to favour one technique over another, and preferentially accumulated experience in one approach. However there has been a recent trend towards the more widespread adoption of SEEG, which has the dual advantage of achieving improved coverage at depth, with lower complication rates.
Curative surgery
Patients with concordant phase 1 investigations, and patients with a robust hypothesis for localisation of EZ following phase 1.5 investigations, may proceed to resective surgery. The goals of resective surgery are to achieve seizure freedom whilst minimising any functional neurological deficit.
Temporal lobe surgery
The standard Anterior Temporal Lobe Resection (ATLR) was first described by Penfield in 1952, and is also known as ‘The Montreal Procedure’12 (Penfield et al, 1952.) This involves an en bloc anterolateral neocortical resection extending from the pole along the superior temporal gyrus to the level of the central sulcus in the non-dominant hemisphere or to the precentral sulcus on the dominant side, which corresponds to 5 or 4.5cm respectively, and across the temporal stem to the collateral sulcus separating the fusiform gyrus from the parahippocampal gyrus. The temporal horn of the lateral ventricle is then entered and the mesial temporal structures, including the hippocampus, parahippocampal gyrus,uncus and typically 4/5 of the amygdala, are resected.
Selective amygdalohippocampectomy
The selective amygdalohippocampectomy is a modification of the standard Montreal procedure, with a more selective removal of the mesial temporal structures, sparing much of the lateral neocortex. The procedure was first proposed by Niemeyer in 1958.13 There have been several technical modifications, including Olivier with the trans-cortical or trans-middle temporal gyrus approach,14 and Yasargil with the trans-Sylvian approach.15 This operation is indicated when pathology is limited to the mesial structures. The difference lies in the approach to the temporal horn of the lateral ventricle. The intraventricular component of the operation, with resection of hippocampus, parahippocampal gyrus, uncus and amygdala, remains the same.
The theoretical advantage of a selective approach is that there is sparing of the lateral neocortex, which may preserve neurocognitive function. This has not been clearly demonstrated with studies that compare selective approaches to the standard ATLR.14,16-18
Extra-temporal surgery
Lesionectomy
Lesions that can produce epilepsy include areas of cortical dysplasia, tumours (low grade, dysembryoplastic neuroepithelial tumours (DNET)),19 areas of cerebral infarction or traumatic injury, and vascular malformations.20 Complete removal of the structural lesion and some of the adjacent cortex yields excellent results. Peri-lesional resection can be guided by use of intra-operative EEG.
Surgery for focal cortical dysplasia and structural vascular abnormalities differs from other lesionectomies due to an indistinct border of the structural abnormality. A total resection is made difficult by horizontal encroachment into eloquent cortex, and vertical encroachment into white matter. For this reason ic-EEG is often performed prior to resection, to guide resection margins and protect eloquent cortex.21
Palliative surgery
Palliative surgery is indicated in patients with medically refractory epilepsy where curative surgery is not possible. This may be because presurgical evaluation has not generated a robust hypothesis for the localisation of a solitary EZ that is amenable to resection. Alternatively the EZ may be widespread or multifocal, or arising from eloquent brain.
Hemispherotomy
Hemispherotomy is indicated in patients with unilateral and wide-spread epilepsy. Common conditions include congenital hemiplegia from a prenatal vascular insult, Sturge-Weber syndrome, hemimegencephaly or diffuse hemispheric cortical dysplasia, Rasmussen encephalitis, hemiconvulsion-hemiplegia-epilepsy, or a sequel of trauma or infection.22
The goal is the disconnection of corpus callosum, internal capsule and corona radiata, mesial temporal structures and frontal horizontal fibres. This may be achieved by the vertical parasaggital approach23 or by the lateral or peri-insular approach,24 depending on surgeons’ preference.
Corpus callosotomy
Corpus callosotomy is indicated in patients with intractable generalised epilepsy where one of the main seizures types are atonic seizures or drop attacks. The goal is the section of the corpus callosum. A standard craniotomy and interhemispheric approach is followed by sectioning of the corpus callosum. The optimal extent of sectioning is not fully understood.25 A partial callosotomy involves sectioning of the anterior two thirds of the corpus callosum from the border of the anterior commissure up to the splenium. Sparing the splenium is thought to reduce the risk of disconnection syndromes. A complete callosotomy is carried through the splenium to the arachnoid of the quadrigeminal cistern.
Vagus nerve stimulation
Vagus nerve stimulation (VNS) is an adjunctive treatment in the management of medically refractory epilepsy in patients who are unsuitable candidates for resective surgery. The mechanism of action is not completely understood, although current evidence points towards a deactivation of the nucleus of the solitary tract, with widespread projections to the dorsal raphe nucleus, locus coeruleus, hypothalamus, thalamus, amygdala and hippocampus.26
Deep brain stimulation
There is a long history of interest in the use of Deep brain stimulation (DBS) for epilepsy control. The postulated mechanism of action is by interrupting the propagation of seizure activity or by increasing the overall seizure threshold. Multiple targets have been put forward, centred in and around the circuit of Papez.27
The current results with DBS for the treatment of epilepsy remain modest, even accounting for the difficult patient group with highly refractory epilepsy.28 Stimulation-related side effects have been reported, most commonly with psychiatric disturb- ances and depression. There is also the possibility of habituation to long-term stimulation.
Non-invasive surgery
There is great interest in less invasive surgical treatments that can generate lesions at depth without requiring the opening of the head. These include MR-guided laser therapy, focused ultrasound, stereotactic radiosurgery and also radiofrequency ablation. A more detailed account of these is given elsewhere.29 More work is needed to demonstrate the ef cacy of these techniques.
References
- Engel J, Jr., McDermott MP, Wiebe S, Lang tt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. Jama. 2012;307(9):922-30.
- Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain : a journal of neurology. 2001;124(Pt 9):1683-700.
- Duncan JS. Selecting patients for epilepsy surgery: synthesis of data. Epilepsy Behavior EB. 2011;20(2):230-2.
- Herskovitz M, Schiller Y. Yield of Non-Invasive Phase 1 Presurgical Evaluation in Drug-Resistant Epilepsy Patients. The Israel Medical Association journal : IMAJ. 2016;18(2):76-9.
- Nowell M, Rodionov R, Zombori G, Sparks R, Winston G, Kinghorn J, et al. Utility of 3D multimodality imaging in the implantation of intracranial electrodes in epilepsy. Epilepsia. 2015;56(3):403-13.
- Blount JP, Cormier J, Kim H, Kankirawatana P, Riley KO, Knowlton RC. Advances in intracranial monitoring. Neurosurgical Focus. 2008;25(3):E18-E.
- Cardinale F. Stereotactic robotic application accuracy is very high in ‘in vivo’ procedures. Stereotactic and Functional Neurosurgery. 2015;93(1):68.
- Cardinale F, Cossu M, Castana L, Casaceli G, Schiariti MP, Miserocchi A, et al Stereoelectroencephalography: surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery. 2013;72(3):353-66; discussion 66.
- Cossu M, Cardinale F, Castana L, Citterio A, Francione S, Tassi L, et al. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: a retrospective analysis of 215 procedures. Neurosurgery. 2005;57(4):706-18; discussion -18.
- Nowell M, Rodionov R, Diehl B, Wehner T, Zombori G, Kinghorn J, et al. A novel method for implementation of frameless StereoEEG in epilepsy surgery. Neurosurgery. 2014;10 Suppl 4:525- 33; discussion 33-4.
- Podkorytova I, Hoes K, Lega B. Stereo-Encephalography Versus Subdural Electrodes for Seizure Localization. Neurosurg Clin N Am. 2016;27(1):97-109.
- Pen eld W, Baldwin M. Temporal Lobe Seizures and the Technic of Subtotal Temporal Lobectomy. Annals of Surgery. 1952;136(4):625-34.
- Niemeyer P, Bello H. Amygdalo-hippocampectomy in temporal lobe epilepsy. Microsurgical technique. Excerpta Medica. 1973;293:20.
- Olivier A. Transcortical selective amygdalohippocampectomy in temporal lobe epilepsy. The Canadian Journal of Neurological Sciences Le journal Canadien des Sciences Neurologiques. 2000;27 Suppl 1:S68-76; discussion S92-6.
- Yasargil MG, Teddy PJ, Roth P. Selective amygdalo-hippocampectomy. Operative anatomy and surgical technique. Advances and Technical Standards in Neurosurgery. 1985;12:93-123.
- Adada B. Selective amygdalohippocampectomy via the transsylvian approach. Neurosurg Focus. 2008;25(3):E5.
- Beaton AE, Durnford A, Heffer-Rahn PE, Kirkham F, Grif n A, Gray WP. Transsylvian selective amygdalohippocampectomy in children with hippocampal sclerosis: seizure, intellectual and memory outcome. Seizure. 2012;21(9):699-705.
- Spencer D, Burchiel K. Selective amygdalohippocampectomy. Epilepsy Research and Treatment. 2012;2012(382095):382095-.
- Nowell M, Miserocchi A, McEvoy AW. Tumors in Epilepsy. Seminars in Neurology. 2015;35(3):209-17.
- Frater JL, Prayson RA, Morris IH, Bingaman WE. Surgical pathologic ndings of extratemporal-based intractable epilepsy: a study of 133 consecutive resections. Archives of Pathology & Laboratory Medicine. 2000;124(4):545-9.
- Roper SN. Surgical treatment of the extratemporal epilepsies. Epilepsia. 2009;50 Suppl 8 (SUPPL. 8):69-74.
- De Ribaupierre S, Delalande O. Hemispherotomy and other disconnective techniques. Neurosurgical Focus. 2008;25(3):E14-E.
- Delalande O, Bulteau C, Dellatolas G, Fohlen M, Jalin C, Buret V, et al. Vertical parasagittal hemispherotomy: surgical procedures and clinical long-term outcomes in a population of 83 children. Neurosurgery. 2007;60(2 Suppl 1):ONS19-32; discussion ONS.
- Villemure JG, Mascott CR. Peri-insular hemispherotomy: surgical principles and anatomy. Neurosurgery. 1995;37(5):975-81.
- Jea A, Vachhrajani S, Johnson KK, Rutka JT. Corpus callosotomy in children with intractable epilepsy using frameless stereotactic neuronavigation: 12-year experience at the Hospital for Sick Children in Toronto. Neurosurgical Focus. 2008;25(3):E7-E.
- Fornai F, Ruffoli R, Giorgi FS, Paparelli A. The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation. The European Journal of Neuroscience. 2011;33(12):2169-78
- Wu C, Sharan AD. Neurostimulation for the treatment of epilepsy: a review of current surgical interventions. Neuromodulation : Journal of the International Neuromodulation Society. 2013;16(1):10-24; discussion
- Fridley J, Thomas JG, Navarro JC, Yoshor D. Brain stimulation for the treatment of epilepsy. Neurosurgical Focus. 2012;32(3):E13-E.
- Nowell M, Miserocchi A, McEvoy AW, Duncan JS. Advances in epilepsy surgery. Journal of Neurology, Neurosurgery, and Psychiatry. 2014;85(11):1273-9.

