Abstract
Transient global amnesia (TGA) is a clinically defined syndrome of acute hippocampal dysfunction lasting several hours. Its pathophysiology remains elusive, and current hypotheses favour a non-epileptic cause. But hypothetically TGA might share a common mechanism with its closest mimic, namely transient epileptic amnesia. We will look at a few arguments that could explain why TGA is possibly an epileptic phenomenon and maybe even a form of non-convulsive status epilepticus.
Introduction
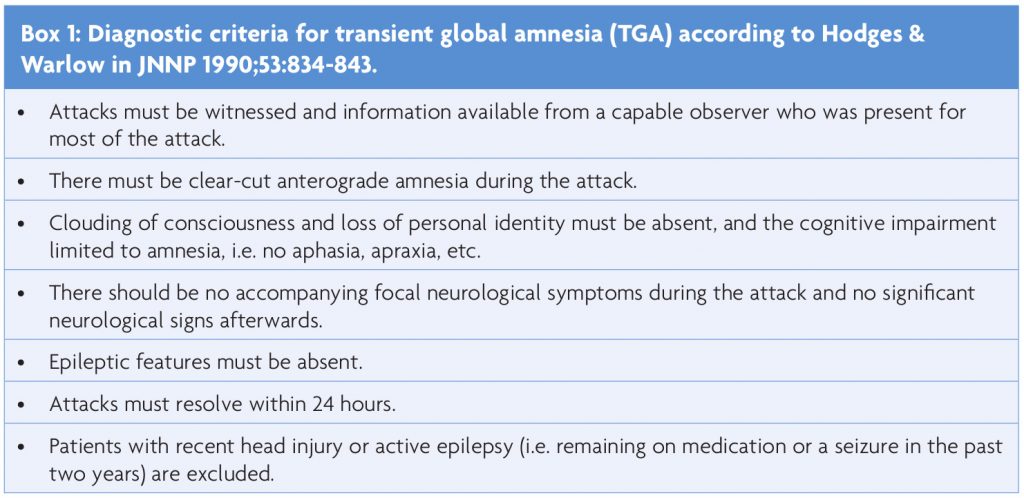
Consistent with our knowledge of memory formation, transient global amnesia (TGA) is a temporary loss of hippocampal function lasting several hours. Patients, typically between 50 and 80 years of age, are suddenly unable to encode new information, without impairment of attention, self-identity or previously learned skills such as driving or using their phone. The neurological examination is unremarkable. They realise that something is wrong with them, become anxious and repeatedly ask the same questions, as they cannot memorise the answer. The whole episode resolves spontaneously leaving only a memory gap of a few hours.1 “TGA does not increase the long-term risk of cerebrovascular events, seizures, or cognitive impairment”.2 The chance of re-occurrence is minimal3 (estimated to be <10% annually), there are no known means of prevention or treatment, and due to its benign nature there is no need for further investigations. Box 1 summarises the clinical TGA criteria based on the work of Hodges & Warlow.4
Numerous hypotheses regarding pathophysiology have been put forward, but controversy remains and there is no clear winner yet. Imaging data are not consistent with the notion of TGA as a form of transient ischaemic attack (TIA), and the subsequent risk of stroke is not increased. But ‘ischaemic amnesia’ is an important TGA mimic, and hypothetically TGA might share a common mechanism with another mimic, namely transient epileptic amnesia (TEA), see below. Several studies have found a statistical association between migraine and TGA. Is TGA a form of aura due to cortical spreading depression? But, if so, why do the typical age groups differ, and why are there no other migraine aura symptoms? Venous congestion of the hippocampus due to a Valsalva manoeuvre has also been proposed as a mechanism and emotional or physical factors can trigger episodes, see e.g. Spiegel1 and Quinette3 et al for review.
Based on my previous work5 and further study of the literature on TGA the following questions will be discussed in this paper:
1) Are the Hodges & Warlow4 diagnostic criteria for TGA specific enough to exclude the risk of missing a mimic such as TEA or ischaemic amnesia?
2) In a comprehensive review of non-convulsive status epilepticus (NCSE), Kinney et al stated that TGA can be readily distinguished from NCSE.6 Does this statement really apply: Could TGA not be a form of NCSE or maybe a post-ictal phenomenon?
When trying to answer these questions, we will also look at the role – and limitations – of EEG in the diagnostic process, and discuss the issue of diffusion-weighted MRI (DWI) lesions in the hippocampus due to amnestic episodes.
Two important mimics of TGA
Michel et al7 published a retrospective case series of patients with amnesia as the predominant presenting symptom. They utilised hospital records and in particular their large stroke data base (3804 patients at the time) to identify clinical clues that might differentiate certain forms of acute ischaemic stroke and transient ischaemic attacks (TIA) presenting predominantly with amnesia (ischaemic amnesia) from transient global amnesia (TGA).
They identified 13 patients who met their inclusion criteria and also had witnessed episodes. The gold standard for the diagnosis of ischaemic amnesia (as opposed to TGA), was the DWI pattern seen in MRI scans (see below).
In 7 out of these 13 cases it was difficult if not sometimes impossible to distinguish them from TGA on a purely clinical basis. Clues pointing to ischaemic amnesia were: minor additional cognitive signs (executive deficits and mild anomia), minor neurological signs and/or an unusual duration of amnesia (<1 and over 24 hours). During long-term follow-up, cognitive assessment demonstrated persistent amnesia in 4/13 patients. All but 1 of the 13 patients demonstrated DWI lesions distinct from the pattern that is thought to be characteristic for TGA. This one patient demonstrated an isolated hippocampal lesion (20 hours after onset) that “was brighter and more linear than the usual punctuate TGA lesions and showed Gadolinium uptake on repeat MRI at eight days, leading to the diagnosis of ischaemic amnesia”. The authors concluded that although ischaemic amnesia could mimic TGA, it is a “rare” occurrence.
But the true rate of underdiagnosis (missing ischaemic stroke or TIA and diagnosing TGA instead) could not be estimated, as less than 25% of the 164 clinically diagnosed TGA patients underwent magnetic resonance imaging with DWI. Given the potential long-term consequences of amnesia, the threshold for an MRI scan with DWI sequences in acutely amnesic patients should be low, particularly in scenarios such as an unusual duration of amnesia, the presence of further cognitive or neurological signs and/or high vascular risk factor load.7
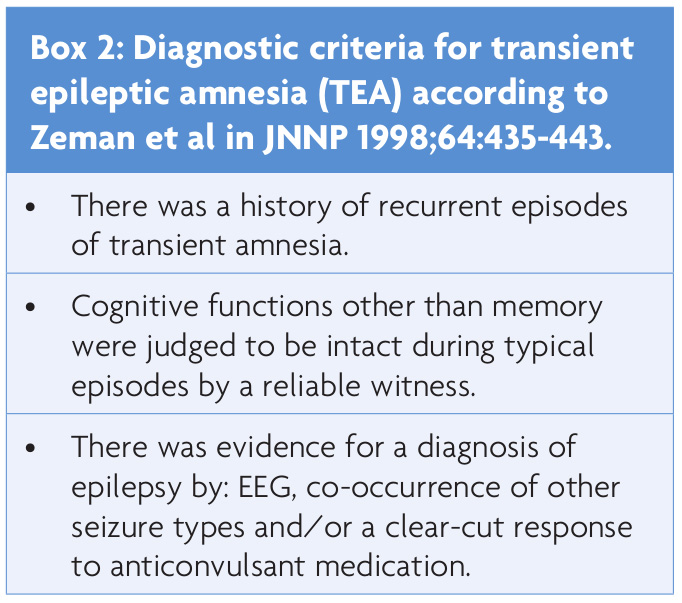
Lanzone et al8 retrospectively analysed clinical and other data of 83 patients with an abrupt occurrence of amnesia that was initially diagnosed as TGA in their emergency department. The aim of the study was to evaluate the accuracy of TGA diagnosis versus the diagnosis of transient epileptic amnesia (TEA) – one of the most relevant TGA mimics. The criteria for TEA according to Zeman9 are summarised in box 2. All patients received an MRI scan and a standard EEG, and additionally a 24-hour ambulatory EEG, if the standard recording was normal or of borderline significance. Using EEG data and the other Zeman criteria, 15 out of 83 patients (18%) were diagnosed with definite TEA (instead of TGA), and 4 more with possible epilepsy.
Does this mean that every amnesia patient needs an EEG and maybe even an additional 24-hour recording because TEA is such a common TGA mimic? Reading between the lines, it seems that a more thorough history and stricter adherence to established diagnostic criteria could have avoided a number of misdiagnoses and diagnostic procedures (but probably not all). For instance, recurrent amnestic episodes, a duration <1 hour, additional cognitive deficits or onset on awakening were good clues that it was not a case of TGA (but TEA instead).
These two case series illustrate once again that despite the unknown pathophysiology of TGA, the diagnostic criteria are quite robust and helpful in recognising mimics that do not have such a benign prognosis. However, they do not exclude all cases with an ischaemic or epileptic aetiology, and these alternative diagnoses should always be considered.
MRI in TGA
Numerous case series have established that the MRI hallmark of TGA are singular ‘punctate’ uni- or bilateral hippocampal DWI lesions that are most common 24-72 hours after onset – at a time when the symptoms have already resolved. These specific hippocampal lesions were rarely seen in the hyperacute phase (2/31), “but all became visible regularly at 48 hours” in 26 of 31 TGA cases in a seminal paper on this topic.10 DWI lesions outside the hippocampus, not ‘punctate’ and/or not delayed (<24 hours) are most likely of ischaemic origin.11 All lesions, in TGA or ischaemia, should be mirrored by a corresponding signal in the ADC sequences. The hippocampal location of these DWI lesions in TGA should not come as a surprise. The hippocampus is basically the cornerstone of memory formation. But how do we explain the delayed appearance 24-72 hours after onset? Clearly, this sets TGA apart from lesions of ischaemic origin that appear within a few hours (at most). DWI lesions reflect – in simplified terms – a disruption of cellular osmotic homeostasis and are potentially reversible, if the triggering factor is reversed fast enough. We could hypothesise that the mechanism (at present unknown) triggering amnesia, also triggers some form of inhibitory mechanism that reverses the amnesia, but does not stop soon enough and so temporarily leads to a disruption of osmotic homeostasis and hence a delayed DWI lesion (which is small enough ‘to be seen but not heard’). This scenario is akin to what we can postulate for Todd’s paresis and leads up the next section.12
Could transient global amnesia be an epileptic phenomenon?
There are several theories regarding the pathophysiology of TGA, but none has stood the test of time (yet).1 The currently employed diagnostic criteria are an empirical construct that does not reflect a particular pathophysiology (but implies a particularly good prognosis).4 So, could transient global amnesia be an epileptic phenomenon? If the answer is ‘yes’, then it might actually be a benign form of NCSE.
There are several case series that reported a lack of epileptiform activity in standard EEG recordings during and after episodes of TGA.3 A ‘standard’ EEG is a surface recording that lasts about 20 minutes. Although this method has excellent specificity (94.7%), its sensitivity is very low (17.3%) as defined by the presence of epileptiform activity.13 One reason is the duration of the EEG. Sensitivity increases with longer recordings, e.g. 53.3% epileptiform abnormalities in standard versus 100% for the 24-hour EEG in the case series of Lanzone.8 Another reason is that epileptiform activity in many cases literally stays below the surface: “90% of cortical spikes with a source area of >10cm2 produced scalp EEG spikes, whereas only 10% of cortical spikes having <10cm2 of source area produced scalp potentials. Intracranial spikes with <6cm2 of area were never associated with scalp EEG spikes”.14 So, all in all, a normal ‘standard’ EEG would not necessarily exclude epileptic activity in the hippocampus during TGA.

We also have to consider the possibility that the disruption of memory formation is due not to ongoing epileptic activity, but rather a post-ictal phenomenon with a normal EEG. The following case report15 might point in that direction: “a brief (<1 minute) burst of left temporal spikes, during which the patient was unresponsive to speech, was followed by normalisation of the EEG and a 10-minute period of amnesia characterised by repetitive questioning about recent events”.
Conclusions
There are (a few) important TGA mimics, see box 3.16 Strict adherence to diagnostic criteria, i.e. painstakingly excluding cognitive deficits beyond pure amnesia and minor neurological abnormalities, will help to identify mimics such as stroke, TIA or TEA. It cannot be stressed enough – in the opinion of this author – that the term ‘transient global amnesia’ is not a generic label that can be applied to every and any amnestic episode. In many older case reports17 the patients actually suffered from what we now call ‘transient epileptic amnesia’ instead of TGA (see boxes for distinct diagnostic criteria). There are some factors beyond the diagnostic criteria that have to be kept in mind: Mild headache, dizziness or nausea/vomiting are not exclusion criteria for TGA, and a certain degree of temporary retrograde amnesia is typical.4 In patients younger than 50 and older than 80 years of age, those with recurrent attacks, atypical duration of amnesia (<1 and longer than 24 hours) and/or episodes on awakening, other differential diagnoses than TGA have to be considered.3 As the duration of TEA and TGA does not always differ significantly (e.g. a median of three for TEA versus four hours for TGA in Lanzone8), the distinction between the two becomes more challenging after a first episode. If there is uncertainty regarding a diagnosis of TGA, then obtain an MRI as soon as possible (do not bother with a CT scan) to exclude non-characteristic DWI and other lesions. An EEG has to be ordered in some cases, but bear its low sensitivity in mind.
In conclusion, the pathophysiology of TGA remains unknown. A benign ictal (NCSE) or a post-ictal phenomenon should be considered as possible causes.
But questions remain: At present, we do not know enough about possible ‘subtypes’ of TGA.18 Is TGA different if we see a right- instead of left-sided DWI lesion? In clinical practice we unwittingly test hippocampal function of the dominant hemisphere when evaluating TGA patients, because we primarily judge retention of verbal information. But we should always try to include a visuospatial (non-verbal) task to test the contra-lateral hippocampus: Have the patient copy a geometric design, and test at regular intervals whether (s)he can reproduce it from memory.
Also, the putative TGA mechanism would have to account for a way to explain bilateral DWI lesions in some cases and why it usually occurs only once, something we would not necessarily expect in focal hippocampal seizures or a mechanism akin to Todd’s paresis. – The search and debate will have to continue.
References
- Spiegel DR, Smith J, Wade RR, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat 2017;13:2691-2703.
- Arena JE, Brown RD, Mandrekar J, Rabinstein AA. Long-term outcome in patients with transient global amnesia: a population-based study. Mayo Clin Proc 2017;92(3):399-405.
- Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain 2006;129:1640-1658.
- Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry 1990;53:834-843.
- Eschle D, Wieser HG. Could transient global amnesia be an epileptic phenomenon? Swiss Arch Neurol Psychiatry 2009;160:73-76.
- Kinney MO, Craig JJ, Kaplan PW. Non-convulsive status epilepticus: mimics and chameleons. Pract Neurol 2018;18:291-305.
- Michel P, Beaud V, Eskandari A, et al. Ischemic amnesia. Causes and outcome. Stroke 2017;48:2270-2273.
- Lanzone J, Ricci L, Assenza G, et al. Transient epileptic and global amnesia: real-life differential diagnosis. Epilepsy Behav 2018;88:205-211.
- Zeman A, Boniface S, Hodges J. Transient epileptic amnesia: a description of the clinical and neuropsychological features in 10 cases and a review of the literature. J Neurol Neurosurg Psychiatry 1998;64:435-443.
- Sedlaczek O, Hirsch JG, Grips E, et al. Detection of delayed focal MR changes in the lateral hippocampus in transient global amnesia. Neurology 2004;62:2165-2170.
- Förster A, Griebe M, Gass A, et al. Diffusion-weighted imaging for the differential diagnosis of disorders affecting the hippocampus. Cerebrovasc Dis 2012;33:104-115.
- Löscher W, Köhling R. Functional, metabolic, and synaptic changes after seizures as potential targets for antiepileptic therapy. Epilepsy Behav 2010;19:105-113.
- Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol 2016;23:455-463.
- Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia 2005;5:669-676.
- Butler CR, Graham KS, Hodges JR, et al. The syndrome of transient epileptic amnesia. Ann Neurol 2007;61:587-598.
- Bartsch T, Butler C. Transient amnesic syndromes. Nat Rev Neurol 2013;9(2):86-97.
- Deisenhammer E. Transient global amnesia as an epileptic manifestation. J Neurol 1981;225:289-292.
- Stracciari A. Transient global amnesia and transient topographical amnesia. An observation favoring the hypothesis of a common pathogenesis. J Neurol 2003;250:633-634.
