Coming up in the Movement Disorders Series
Over the next few editions, we will run a wide-ranging series of clinical movement disorder articles. There is a Scottish theme to the first three articles with the authors hailing from Edinburgh, Dundee and Glasgow.
In their article titled ‘Functional tics, the pandemic and social media’ Neil Ramsay, Vicky Marshall and Jon Stone will make sense of the upsurge in functional tics reported during the COVID-19 pandemic and discuss whether, as widely reported in the press, social media had a role in this.
In clinical neurosciences, we love using new technologies to revive old ideas and surgical thalamotomy is one of the oldest ways of managing tremor. Tom Gilbertson and Sadaquate Khan have been instrumental in the recent establishment of an MR guided focused ultrasound service at Ninewells Hospital, Dundee. In their paper, they will outline the latest evidence for the use of MR guided focused ultrasound in tremor and discuss where this treatment may go.
Recognising and confidently diagnosing focal dystonias can be challenging for the general neurologist. In this first article in our series, entitled ‘A Clinical Approach to Focal Dystonias’ Sacha Gandhi, Dave Anderson and I discuss the common presentations of adult-onset cranio-cervical and limb dystonias.
This movement disorder series will continue later this year with articles on pathophysiology of non-motor aspects of Parkinson’s disease and other fascinating topics. I encourage you strongly to read these articles and thank you for your time.
Ed Newman, ACNR’s Movement Disorders Editor.
Abstract
Dystonia is a hyperkinetic movement disorder (HMD), characterised by sustained or intermittent involuntary muscle contractions resulting in abnormal postures and/or movements [1]. Although primary dystonia has an estimated prevalence of 16 per 100,000 [2], the diagnosis may be delayed, due to its clinical heterogeneity, the lack of objective biomarkers and the potential for pseudodystonic conditions to mimic it [1,3]. We provide an overview of the classification and common subtypes of focal dystonia, focusing on the clinical phenomenology and diagnosis.
Clinical features of dystonia
Dystonia is diagnosed clinically, based upon its phenomenology. In dystonia, involuntary co-contraction of agonist and antagonist muscles gives rise to abnormal postures and movements. Dystonic movements are often stereotyped, patterned and twisting, and may be tremulous. Dystonia is often exacerbated by voluntary movement or posture and may be task-specific. Over time, however, it may manifest with less specific movements or emerge at rest. A core clinical feature of dystonia is the geste antagoniste, a so-called alleviating manoeuvre or ‘sensory trick’ that transiently improves the movement or posture. The term ‘sensory trick’ may be a misnomer. Although the exact mechanism remains elusive, it is likely that motor output plays a greater role in alleviating the dystonia than sensory input from the periphery. Other clinical hallmarks include mirror dystonia and motor overflow. Mirror dystonia describes abnormal posturing or movement elicited by activities performed by the contralateral unaffected body region. Patients with writer’s cramp, for example, may exhibit a mirror dystonia of their affected hand when writing with the contralateral unaffected hand. Motor overflow occurs when involuntary muscle contractions spread from the primary site of dystonia to a contiguous unaffected body region [1]. Non-motor features of dystonia, including neuropsychiatric comorbidities, pain, impaired cognition and sleep, contribute to impaired quality of life [4]. We will use the term pseudodystonia to describe a heterogeneous group of conditions with abnormal postures and/or repetitive movements that may mimic dystonia. These conditions have phenomenological features and underlying aetiologies which are not compatible with dystonia [1].
Classification of dystonia
Classification schemes for dystonia have been proposed and refined several times since Oppenheim first described the condition in 1911. The current classification scheme categorises dystonia into two distinct axes: 1) clinical characteristics and 2) aetiology. The clinical axis incorporates age at onset (infancy, childhood, adolescence, early and late adulthood); the affected body regions (focal, segmental, multifocal and generalised); temporal pattern (static or progressive; persistent, paroxysmal, or diurnal; action-specific), and any additional associated features including other movement disorders. The second axis, aetiology, encompasses anatomical changes on imaging or pathology and the pattern of inheritance [1].
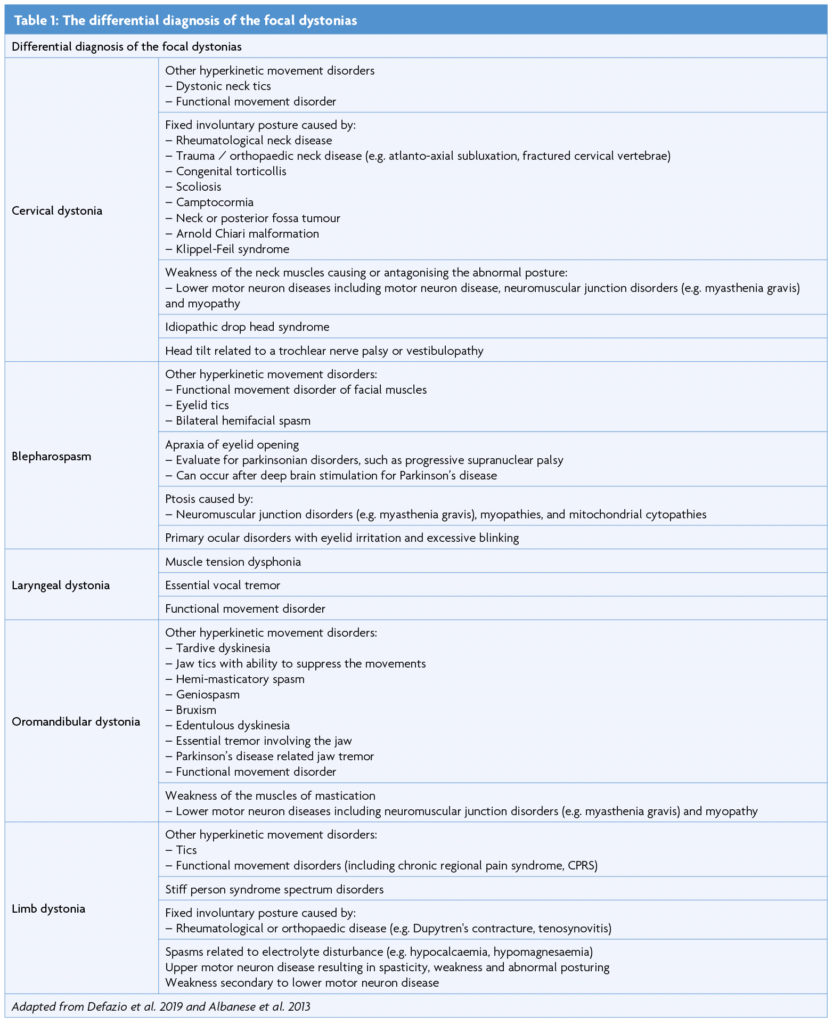
Focal dystonia refers to dystonia confined to a single body region. It almost always affects adults, and typically involves the face, neck or upper limbs. Diagnostic criteria have recently been validated for blepharospasm [5], and expert recommendations may aid in the diagnosis of laryngeal [6], cervical, oromandibular, and limb dystonia [3]. These emphasise basing the diagnosis on its positive phenomenological features, supported by the presence of a geste antagoniste. Negative features suggestive of dystonia mimics should be excluded. These include fixed postures, neuromuscular weakness, and the ability to voluntarily suppress spasms, suggestive of tics [3]. Different types of focal dystonias may co-exist. Typical combinations include cervical dystonia (CD) with writer’s cramp, CD with Meige syndrome, and CD with laryngeal dystonia (LD).
Adult onset focal dystonias
Cervical dystonia
Cervical dystonia (CD), by far the most common adult onset focal dystonia (AOFD), has a predilection for Caucasian females in their fourth decade [7]. Patients often report a gradual onset neck pain, stiffness or ‘pulling’ sensation, followed by abnormal head and neck postures and movements. However, dystonia with an acute onset has been described and may be associated with trauma [8]. The Col-Cap concept classifies dystonia according to the position of the cervical spine (collum-type, CACOL), the position of the head (caput-type, ACAP), or both (shifts). This categorises CD into 11 distinct subtypes: torticollis, torticaput, anterocollis, anterocaput, retrocollis, retrocaput, laterocollis, laterocaput, lateral shift, forward sagittal shift and posterior sagittal shift (both retrocollis and anterocaput combined) [9]. A significant proportion of patients may have associated head tremor [7], jerking or phasic movements. The dystonia may be exacerbated by anxiety and transiently ameliorated by a geste antagoniste, such as touching the lower face. CD is associated with psychological and functional disability, together with significant pain in up to 70% of patients [10], which may impact upon activities of daily living (ADLs) such as reading, watching television and driving.
The majority of CD is idiopathic but genetic and acquired causes are recognised. It may occur as an acute dystonic reaction or tardive syndrome, or may arise secondary to parkinsonian disorders, structural lesions, trauma or autoimmune disease [11]. Underlying focal lesions can localise to the brainstem, cerebellum, basal ganglia or cervical spinal cord [12]. CD mimics include neck tics, neck weakness from neuromuscular disease, and fixed involuntary postures secondary to rheumatological or orthopaedic disease, as illustrated in Table 1.
CD has been rarely described in patients with genetic mutations typically associated with generalised dystonias, such as DYT-TOR1A but more commonly with DYT-THAP17. Craniocervical dystonia, including isolated CD, has been reported in patients with DYT-GNAL7,13, DYT-ANO314 and CIZ115, although the role of the last two mutations in CD requires further confirmation.
Blepharospasm
Blepharospasm (BSP), one of the most common forms of AOFD, has a mean age at diagnosis of 55.7 years [16]. According to recently validated diagnostic criteria [5], blepharospasm is characterised by bilateral, synchronised and stereotyped orbicularis oculi spasms, leading to eyelid narrowing or closure. A geste antagoniste provides further support for diagnosis. If a geste antagoniste is absent, lack of suppressibility or excessive blinking may enhance diagnostic sensitivity and specificity [5]. Applying these criteria may accurately differentiate BSP from mimics, including tics, ptosis secondary to neuromuscular disease and apraxia of eyelid opening (often associated with parkinsonian disorders such as progressive supranuclear palsy) [5]. Severe BSP may render patients functionally blind, impacting upon their ability to read, watch television, independently ambulate and drive.
The pathogeneisis of BSP is multifactorial, with both environmental and genetic factors. Ocular disease, such as dry eye and keratoconjunctivitis, often precede its development, and caffeine may exert a protective effect [16-17]. Although most cases are sporadic, between 11-30% of patients with BSP disclose a family history of dystonia, lending support to an underlying genetic predisposition [17].
Laryngeal dystonia
Laryngeal dystonia (LD) is a rare task-specific focal dystonia, in which laryngeal dystonic spasms selectively impair speech. The condition may present insidiously or abruptly, often in female patients in their forties, following a period of major stress or an upper respiratory tract infection [18]. Adductor (ADLD) and abductor LD (ABLD) are the most common subtypes, although other variants have been described (mixed LD, adductor respiratory dystonia, and singer’s dystonia). Speech has a strained, ‘strangled’ quality in ADLD, due to hyperadduction of the vocal cords, but is ‘whispering’ with breathy pauses in ABLD [18-19]. By interfering with effective communication, LD may be a socially disabling disorder with considerable psychosocial impact.
In addition to history and neurological examination, speech examination with nasolaryngoscopy is required for a definitive diagnosis [6]. Although dystonic spasms may occur during speech, the larynx should be structurally normal, with functional vocal cords. LD should be differentiated from muscle tension dysphonia (MTD) and essential vocal tremor (VT) although these can co-exist in up to one third of cases. Task-specificity is a core clinical feature of LD and helps to differentiate it from MTD and essential VT. The presence of a geste antagoniste, such as laughing or humming before speaking, further supports a diagnosis of LD.
LD is typically idiopathic, but symptoms may rarely occur secondary to structural brain disease or neuroleptic exposure. Up to 25.3% of patients with LD disclose a family history of dystonia, suggesting a genetic predisposition for a subset of cases. Indeed, LD has been associated with genes typically linked to generalised or segmental dystonia syndromes (DYT-TOR1A, DYT-TUBB4A, DYT-THAP1, DYT-GNAL and DYT-KMT2B) [18]. DYT-THAP1, in particular, may be associated with prominent LD20. DYT-TUBB4A, caused by missense mutation in the TUBB4A gene, is associated with ‘whispering dysphonia’. The clinical phenotype of DYT-TUBB4A is heterogenous, ranging from isolated LD to severe generalised dystonia, accompanied by a ‘hobby horse’ ataxic gait [21].
Oromandibular dystonia
Oromandibular dystonia (OMD) is a focal dystonia involving the lower face, jaw and tongue. It is more common in women, with symptoms generally emerging in the fifth decade [22]. When accompanied by blepharospasm, it is termed Meige syndrome. OMD may be characterised by varying combinations of lip pursing, tongue dyskinesia, and jaw opening, closure, deviation, protrusion or retraction. The dystonia may occur spontaneously or may be task-specific, triggered by speech and mastication. As OMD impacts upon speech and eating, it may be a socially debilitating condition, with significant associated weight loss and impaired quality of life.
OMD may be idiopathic, a tardive phenomenon or a manifestation of an underlying hereditary condition, such as DYT-TOR1A dystonia, X-linked dystonia parkinsonism or neuroacanthocytosis [22]. Embouchure dystonia is a task-specific form of OMD affecting musicians who play brass and woodwind instruments. As illustrated by Table 1, numerous conditions may mimic OMD, including jaw tics, edentulous dyskinesia and jaw tremor, which may be essential or associated with parkinsonian conditions. Other mimics may include geniospasm, an autosomal dominant condition with early onset jaw tremor; hemimasticatory spasm, which may be associated with facial hemiatrophy; and bruxism, characterised by forced jaw closure and dental attrition during sleep or emotional distress. Diagnosis requires eliciting the positive features of dystonia, excluding negative features and identifying a geste antagoniste. Pertinent negative features include non-stereotyped or patterned movements, suppressibility, facial hemiatrophy, temporomandibular joint disorders, masticatory muscle weakness, edentulism or dental attrition, pain and other sensory symptoms, and autosomal dominant inheritance [3].
Limb dystonia
Numerous task-specific limb dystonias have been described, of which writer’s cramp is the most common. These tend to occur in professionals engaging in repetitive highly skilled motor activities over prolonged periods of time. Writer’s cramp affects adults between the ages of 30 to 50 years, presenting with an initial tension within the forearm, which culminates in cramp on writing with abnormal grip and posturing. On holding the pen, the thumb and index fingers develop abnormal postures, with excessive finger flexion, ulnar abduction and wrist flexion. There may be associated forearm supination and more proximal involvement of the arm and shoulder. Tremor accompanies less than one third of cases. The dystonia may remain task-specific or may affect other non-specific movements, resulting in complex writer’s cramp [23].
Isolated adult-onset focal limb dystonia is typically sporadic but may be a manifestation of hereditary dystonic syndromes such as DYT-TOR1A, DYT-THAP1, or DYT-GCH1. Most focal limb dystonias affect the upper limb; leg onset should therefore raise the suspicion of a secondary cause [23]. As focal upper limb dystonias, such as writer’s cramp, typically affect patients between the ages of 30 to 50 years, early onset before the age of 30 years is a clinical red flag. In such patients, genetic counselling and diagnostic testing for TOR1A is indicated [24]. Dystonic limb posturing may be associated with structural lesions of the basal ganglia, thalamus and brainstem, particularly when abrupt in onset or accompanied by additional neurological signs. Limb dystonia may also be the first manifestation of neurodegenerative disease, including Parkinson’s disease, Wilson’s disease, Huntington’s disease, X-linked dystonia parkinsonism (DYT-TAF1) and neuroacanthocytosis. Although neuroleptic exposure typically produces craniofacial dystonia, limb involvement may occur [23]. As in Table 1, numerous conditions may mimic limb dystonia, including tics; functional movement disorders; stiff person syndrome spectrum disorders; orthopaedic or rheumatological disease, resulting in fixed abnormal postures; and neuromuscular weakness [3,23].
Conclusion
The focal dystonias are a rare and heterogenous group of conditions. In view of this, they are often not recognised by primary care physicians. Their phenomenological variability, combined with the potential for pseudodystonic conditions to mimic them, contributes to diagnostic delay and misdiagnosis. This paper provides an overview of the clinical classification and phenomenology of the focal dystonias as an aide to diagnosis. Early recognition of the stereotyped and patterned nature of dystonic movements is fundamental to accurate diagnosis. Expert recommendations can aid in the diagnosis of CD, OMD, LD and limb dystonia, and validated diagnostic guidelines exist for BSP. Video illustrations disseminated in journals and teaching material may assist in pattern recognition and diagnosis. A multidisciplinary approach can facilitate timely diagnosis and treatment, and has become an established part of the diagnostic process in some parts of the United Kingdom.
References
- Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;15;28(7):863-73. https://doi.org/10.1002/mds.25475
- Steeves TD, Day L, Dykeman J, Jette N, Pringsheim T. The prevalence of primary dystonia: a systematic review and meta-analysis. Mov Disord. 2012;27(14):1789-96. https://doi.org/10.1002/mds.25244
- Defazio G, Albanese A, Pellicciari R, Scaglione CL, Esposito M, Morgante F, et al. Expert recommendations for diagnosing cervical, oromandibular, and limb dystonia. Neurol Sci. 2019;40(1):89-95. https://doi.org/10.1007/s10072-018-3586-9
- Stamelou M, Edwards MJ, Hallett M, Bhatia KP. The non-motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain. 2012;135(Pt 6):1668-81. https://doi.org/10.1093/brain/awr224
- Defazio G, Jinnah HA, Berardelli A, Perlmutter JS, Berkmen GK, Berman BD, et al. Diagnostic criteria for blepharospasm: A multicenter international study. Parkinsonism Relat Disord. 2021;91:109-114. https://doi.org/10.1016/j.parkreldis.2021.09.004
- Ludlow CL, Adler CH, Berke GS, Bielamowicz SA, Blitzer A, Bressman SB, et al. Research priorities in spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139(4):495-505. https://doi.org/10.1016/j.otohns.2008.05.624
- LeDoux MS, Vemula SR, Xiao J, Thompson MM, Perlmutter JS, Wright LJ, et al. Clinical and genetic features of cervical dystonia in a large multicenter cohort. Neurol Genet. 2016;2(3):e69. https://doi.org/10.1212/NXG.0000000000000069
- O’Riordan S, Hutchinson M. Cervical dystonia following peripheral trauma–a case-control study. J Neurol. 2004;251(2):150-5. https://doi.org/10.1007/s00415-004-0291-9
- Reichel G, The COL CAP concept, 3rd edition self-publishing company, 2014.
- Avenali M, De Icco R, Tinazzi M, Defazio G, Tronconi L, Sandrini G, Tassorelli C. Pain in focal dystonias – A focused review to address an important component of the disease. Parkinsonism Relat Disord. 2018;54:17-24. https://doi.org/10.1016/j.parkreldis.2018.04.030
- Jinnah HA, Berardelli A, Comella C, Defazio G, Delong MR, Factor S, et al. Dystonia Coalition Investigators. The focal dystonias: current views and challenges for future research. Mov Disord. 2013;15;28(7):926-43. https://doi.org/10.1002/mds.25567
- LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18(1):60-9. https://doi.org/10.1002/mds.10301
- Fuchs T, Saunders-Pullman R, Masuho I, Luciano MS, Raymond D, Factor S, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2013;45(1):88-92. https://doi.org/10.1038/ng.2496
- Charlesworth G, Plagnol V, Holmström KM, Bras J, Sheerin UM, Preza E, et al. Mutations in ANO3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis. Am J Hum Genet. 2012;91(6):1041-50. https://doi.org/10.1016/j.ajhg.2012.10.024
- Xiao J, Uitti RJ, Zhao Y, Vemula SR, Perlmutter JS, Wszolek ZK, et al. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann Neurol. 2012;71(4):458-69. https://doi.org/10.1002/ana.23547
- Hallett M, Evinger C, Jankovic J, Stacy M; BEBRF International Workshop. Update on blepharospasm: report from the BEBRF International Workshop. Neurology. 2008;14;71(16):1275-82. https://doi.org/10.1212/01.wnl.0000327601.46315.85
- Defazio G, Abbruzzese G, Aniello MS, Bloise M, Crisci C, Eleopra R, et al. Environmental risk factors and clinical phenotype in familial and sporadic primary blepharospasm. Neurology. 2011;16;77(7):631-7. https://doi.org/10.1212/WNL.0b013e3182299e13
- Simonyan K, Barkmeier-Kraemer J, Blitzer A, Hallett M, Houde JF, Jacobson Kimberley T, et al; The NIH/NIDCD Workshop on Research Priorities in Spasmodic Dysphonia/Laryngeal Dystonia. Laryngeal Dystonia: Multidisciplinary Update on Terminology, Pathophysiology, and Research Priorities. Neurology. 2021;25;96(21):989-1001. https://doi.org/10.1212/WNL.0000000000011922
- Blitzer A, Brin MF, Simonyan K, Ozelius LJ, Frucht SJ. Phenomenology, genetics, and CNS network abnormalities in laryngeal dystonia: A 30-year experience. Laryngoscope. 2018 Jan;128 Suppl 1(Suppl 1):S1-S9. Epub 2017 Dec 8. https://doi.org/10.1002/lary.27003
- Xiao J, Zhao Y, Bastian RW, Perlmutter JS, Racette BA, Tabbal SD, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 2010;19;74(3):229-38. https://doi.org/10.1212/WNL.0b013e3181ca00ca
- Hersheson J, Mencacci NE, Davis M, MacDonald N, Trabzuni D, Ryten M, et al. Mutations in the autoregulatory domain of β-tubulin 4a cause hereditary dystonia. Ann Neurol. 2013;73(4):546-53. https://doi.org/10.1002/ana.23832
- Scorr LM, Factor SA, Parra SP, Kaye R, Paniello RC, Norris SA, et al. Oromandibular Dystonia: A Clinical Examination of 2,020 Cases. Front Neurol. 2021 Sep 16;12:700714. https://doi.org/10.3389/fneur.2021.700714
- Pont-Sunyer C, Martí MJ, Tolosa E. Focal limb dystonia. Eur J Neurol. 2010 Jul;17 Suppl 1:22-7. https://doi.org/10.1111/j.1468-1331.2010.03046.x
- Albanese A, Barnes MP, Bhatia KP, Fernandez-Alvarez E, Filippini G, Gasser T, Krauss JK, Newton A, Rektor I, Savoiardo M, Valls-Solè J. A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: report of an EFNS/MDS-ES Task Force. Eur J Neurol. 2006 May;13(5):433-44. https://doi.org/10.1111/j.1468-1331.2006.01537.


