Abstract
Sleep disturbance is common following traumatic brain injury (TBI) and can affect recovery course and disrupt rehabilitation. Poor sleep affects neuropsychiatric, behavioural and physical symptoms as well as learning and memory, leading to suboptimal cognitive recovery and prolonged stay in the hospital. Sleep disturbance may also be a marker for more severe injury.
The data on the prevalence of sleep problems following TBI is relatively limited; various studies looking into this have reported overall prevalence rates of between 40 and 70%. Furthermore, TBI patients with sleep problems often have difficulty identifying their symptoms, making it more difficult to establish the true nature of these. Numerous factors contribute including the direct effect of trauma, neuropsychiatric consequences, psychotropic medication and increased risk of primary sleep disorders such as sleep apnoea if inactivity leads to increased weight gain. The relationship between the type or location of injury and sleep disturbance is not well established.
Sleep disturbances commonly identified post-TBI include insomnia, hypersomnia and alterations of the sleep-wake cycle or circadian rhythm. A detailed sleep history from patient and/or carer is vital. Sleep assessment scales including the Epworth Sleepiness Scale are useful alongside review of sleep charts for in-patients. One must consider neuropsychiatric comorbidities including depression, anxiety and pain. Referral to a sleep service for respiratory sleep studies or polysomnography can help to accurately diagnose sleep apnoea, parasomnia or causes of hypersomnia and many sleep disorders have effective therapies.
The key to management is establishing an accurate sleep disorder diagnosis. The treatment of insomnia and circadian rhythm disorder should initially be non-pharmacological, focusing on sleep hygiene and CBT and regulating exposure to natural light. Stimulants may be effective for persistent hypersomnia if respiratory causes have been excluded.
Background
Sleep-wake disturbances (SWDs) are commonly seen in patients who sustain a traumatic brain injury (TBI). For example, Gardani et al1 recently found that 2/3 of their rehabilitation inpatients with severe TBI showed signs of a sleep cycle disturbance, and 50% met the criteria for a sleep disorder. This can occur in the immediate period after the injury and may persist for several years after the event.2,3
SWDs can affect the recovery course and exacerbate other problems commonly seen in the recovery period post-TBI including pain, fatigue, cognitive impairment and psychiatric problems such as anxiety and depression4 – indeed, Rao et al5 found that SWDs in the acute post-injury period were associated with neuro-psychiatric symptoms for the next year. As such, it is important for the clinician managing patients with TBI to be aware of the possible sleep problems that may manifest themselves and how to appropriately investigate and manage them.
What should we be looking for?
Prevalence of sleep disorders varies significantly between studies due to differences in methodology, diagnostic criteria and patient populations; Table 1 highlights the most common disorders and selected prevalence figures.
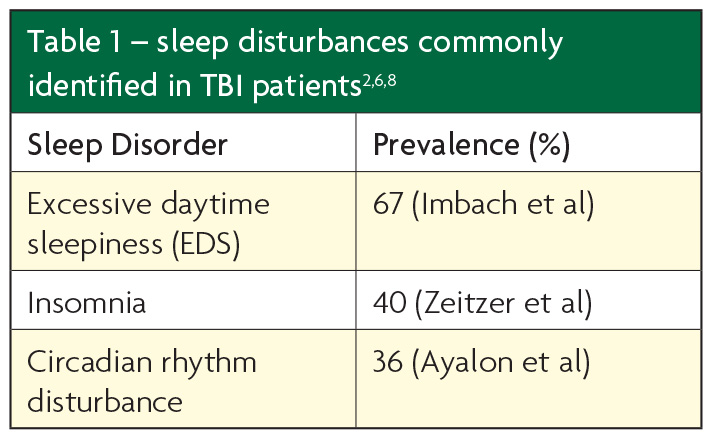
Despite these variations, reduced amount of sleep (insomnia), excessive daytime sleepiness (EDS), increased sleep need (pleiosomnia) and circadian rhythm disturbance are generally the most commonly seen SWDs in TBI patients. It is suggested that in the immediate post-injury phase patients have problems with initiating and maintaining sleep, whereas chronic brain injury patients experience excessive sleep.
Insomnia is likely multifactorial, and may be related to the neural damage itself or neuropsychiatric/neuromuscular sequelae (e.g. depression, pain). Zeitzer et al highlight in their review6 that there are broadly two types of patients in this area; those who report difficulty sleeping without necessarily having objective findings to correlate with this, and those with increased daytime sleepiness and reduced concentration with more investigative correlates.
Pleiosomnia, that is to say increased need for sleep, and EDS have also recently been found to be especially common in TBI patients, with patients requiring on average an extra hour of sleep per 24 hours compared to healthy controls.2 Objectively measured EDS is seen in up to two-thirds of patients and for at least 18 months post-injury, although evidence suggests that patients themselves may not report this. As is seen in insomnia, there is often significant discrepancy between patient description and investigative findings.
Circadian rhythm disturbance can take the form a delayed sleep phase disorder (DSPD)8 or less commonly an irregular sleep wake pattern or free running pattern and may be related to dysfunctional melatonin production. There is a relationship between DSPS and depressive symptoms; whether there is a causal relationship between the two is as yet uncertain.
Sleep apnoea, especially obstructive (OSA), is a common cause of daytime sleepiness in the general population and although it may not be caused directly by brain injury, it may slow down or complicate recovery.10 Risk factors include weight secondary to decreased activity and some psychotropic medications alongside sedative or opioid medication. It is also significant for its implications on driving and long-term cardiovascular risk. It is important to remember this has a well tolerated and cost effective treatment with CPAP therapy.
Asking patients about restless legs as a common secondary cause of insomnia is also important, checking serum ferritin for such patients and replacing if below 45 may be of benefit.
In less common disorders such as parasomnias, the duration of sleep is typically unaffected but patients (or more likely, carers) may complain of sleep-walking or other unusual activities during sleep; however, there is debate regarding the true prevalence and clinical presentation of parasomnias after brain injury. Narcolepsy with cataplexy, another cause of excessive sleepiness, is also occasionally described but there is little evidence of a significantly increased risk following TBI. Interestingly, transient hypocretin deficiency is seen after TBI which provides one mechanism for hypersomnia.
How should we investigate?
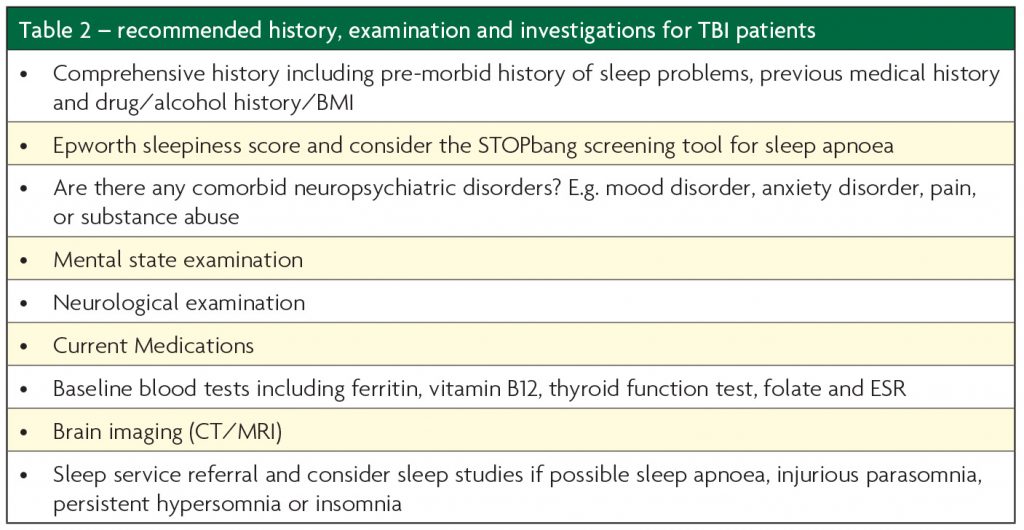
The investigative process should be guided by clinical judgement based on the symptoms present and level of neuropsychiatric impairment in the patient; a standard approach often suffices. Simple self-report methods such as the Epworth Sleepiness Scale (ESS) and Pittsburgh Sleep Quality Index may be of use in less cognitively impaired patients but it should be considered that TBI patients tend to under-report excessive sleepiness and insomnia.2,6 Added value can come from asking the bedpartner or spouse to complete the ESS where possible. Assessment of BMI, caffeine, nicotine and alcohol intake alongside regular medications should also be a standard part of the clinician’s initial workup. As such, a sleep history and typical 24 hours from the patient and/or carer should be part of all TBI assessments.
Sleep charts, which can be completed by nursing staff for patients in inpatient facilities, are useful to quantify the amount and timing of sleep and thus can suggest a need for further investigation or management. Furthermore, sleep charts over time can be used to more objectively monitor response to treatment especially for disorders such as insomnia or hypersomnia.
There is widespread availability of domiciliary respiratory sleep studies to screen for sleep apnoea. Video-Polysomnography (PSG) and occasionally actigraphy are objective tests for assessing the amount and quality of a patient’s sleep but are limited by availability in some settings. Where they are available these tests, along with Multiple Sleep Latency Testing (MSLT) are the gold standard for pathological hypersomnia and, have utility in investigating disorders where the aetiology is not elicited through simple measures or self-report questionnaires.12 They can also help differentiate sleep disorders from fatigue, which is also common post-TBI and can confuse the picture.
It should be considered that such complaints as insomnia or excessive sleepiness may be symptoms of other underlying primary sleep disorders (such as OSA or restless syndrome), or part of psychiatric disorders such as depression and PTSD or other medical comorbidities. Screening or further investigation for these may therefore prove useful, as would referral on to appropriate clinicians for further assessment and management.
Once we’ve identified a problem – what next?
The key to management lies in integrating the sleep disorder itself with comorbidities and contributory factors, and tackling each of these appropriately. Mood disorders and pain may be difficult to manage but any improvement in these may yield direct benefits to sleep. Equally improving sleep is shown to improve mood and pain scores.
At a conservative level sleep hygiene focus is often a useful first step in management, particularly for patients presenting with insomnia, hypersomnia or circadian rhythm disturbances. This may consist of simple measures such as reinforcing a regular sleep schedule, avoiding stimulant drinks like tea/coffee and limiting night-time stimulation from electronic devices with bright screens. Non-pharmacological methods such as CBT should be considered first-line for insomnia (CBT-I) with evidence for benefit in primary and comorbid insomnia. For hypersomnia, reviewing sedative medication and maintaining simple sleep charts over a two week period should be considered initially to highlight any contributory lifestyle factors.
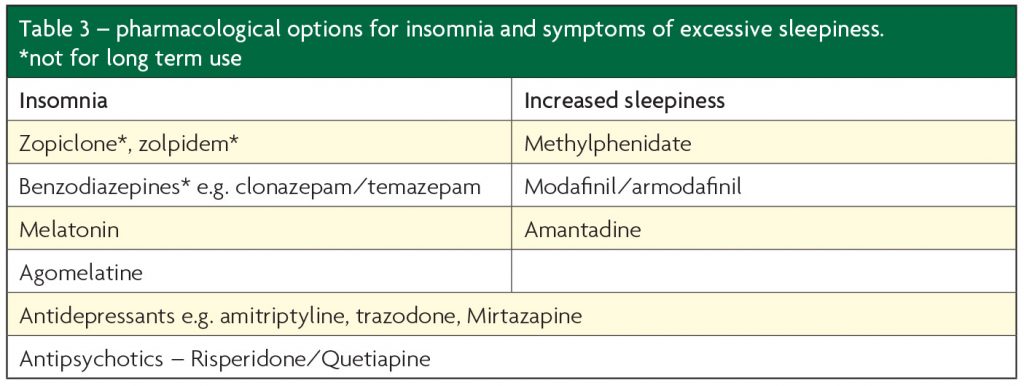
There are numerous pharmacological options available for insomnia and EDS (see Table 3) and medications may be useful in a range of underlying disorders, such as antidepressants for depression/anxiety disorders and low dose antipsychotics in aggressive patients.
The literature surrounding the use of these medications in TBI populations is however quite limited and their use should be evaluated in terms of the benefit they impart versus any side-effects they yield – particularly any cognitive or behavioural changes.
From our clinical experience, we have perceived more benefit from the use of low-doses of trazodone (e.g. 75mg) and mirtazapine (e.g. 15mg) for insomnia. However, mirtazapine can aggravate restless legs so this should be screened for first. Benzodiazepines should be used with caution and only for short periods of two weeks or less, they have very limited evidence base for chronic insomnia. They may contribute to symptoms of sleep apnoea as well as worsening daytime tiredness. Likewise, Z-drugs (zopiclone, zolpidem) should be avoided where possible.
Finally, if the standard measures to improve sleep disturbance fail, referral to a specialist sleep service is important as these clinicians may be able to elicit the true nature of the apparent disorder in order to guide further treatment. They may also provide other alternatives to medications such as continuous positive airway pressure (CPAP) for OSA.
Is there a need to screen?
Given that SWDs are so common in this population, we would argue that any patient presenting post-TBI should be screened for such a disturbance.13 There are, however, currently several questions to address before this can be implemented as standard:
- Are there any risk factors within the TBI patient group that yield an even higher likelihood of SWD being present, such as TBI severity, area of injury, type of injury or presence/duration of unconsciousness?
- Is it logistically and economically viable to screen for SWDs in the context of their prevalence and importance in this population group?
- What are the most effective management methods for SWDs in these patients, and does effective management lead to better overall long-term outcomes? There is evidence suggesting that such measures do indeed improve cognitive and behavioural outcomes14 but further work is needed.
- What are the biological mechanisms behind the high prevalence of SWDs in the TBI population? There are several hypotheses, such as the role of sleep in clearance of metabolic waste products,15 further research into which may guide future management options (e.g. correcting a neurotransmitter imbalance) or allow better prediction of those more at risk of SWD.
If it is deemed to be viable, work in these areas may facilitate the development of a robust, evidence-based screening approach or set of guidelines for the assessment and management of post-TBI SWDs.
References
- Gardani M, Morfiri E, Thomson A, O’Neill B, McMillan TM. Evaluation of Sleep Disorders in Patients With Severe Traumatic Brain Injury During Rehabilitation. Archives of Physical Medicine & Rehabilitation 2015;96:1691-1.
- Imbach LL, Büchele F, Valko PO, Li T, Maric A, Stover JF, Bassetti CL, Mica L, Werth E, Baumann CR. Sleep-wake disorders persist 18 months after traumatic brain injury but remain under recognised. Neurology 2016;86:1945-9.
- Kempf J, Werth E, Kaiser PR, Bassetti CL, Baumann CR. Sleep-wake disturbances 3 years after traumatic brain injury. Journal of Neurology, Neurosurgery & Psychiatry 2010;81:1402-1405.
- Ouellet M-C, Beaulieu-Bonneau S, Morin CM. Sleep-wake disturbances after traumatic brain injury. The Lancet Neurology 2015;14:746-757.
- Rao V, McCann U, Han D, Bergey A, Smith MT. Does acute TBI-related sleep disturbance predict subsequent neuropsychiatric disturbances? Brain Injury 2014;28:20-6.
- Zeitzer JM, Friedman L, O’Hara R. Insomnia in the context of traumatic brain injury. Journal of Rehabilitation Research and Development 2009;46:827-36.
- Shekleton JA, Parcell DL, Redman JR, Phipps-Nelson J, Ponsford JL, Rajaratnam SMW. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology2010;74:1732-8.
- Ayalon L, Borodkin K, Dishon L, Kanety H, Dagan Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology 2007;68:1136-1140.
- Castriotta RJ, Murthy JN. Sleep disorders in patients with traumatic brain injury. CNS Drugs 2011;25:175-85.
- Sofela A, Sakel M. Prevalence and diversity of sleep impairments in TBI, a systematic review. Archives of Physical Medicine and Rehabilitation 2014;95:e48.
- Wilde MC, Castriotta RJ, Lai JM, Atanasov S, Masel BE, Kuna ST. Cognitive impairment in patients with traumatic brain injury and obstructive sleep apnea. Archives of Physical Medicine & Rehabilitation 2007;88:1284-8.
- Mollayeva T, Kendzerska T, Colantonio A. Self-report instruments for assessing sleep dysfunction in an adult traumatic brain injury population: A systematic review. Sleep Medicine Reviews 2013;17:411-23.
- Mollayeva T, Colantonio A, Mollayeva S, Shapiro CM. Screening for sleep dysfunction after traumatic brain injury. Sleep Medicine 2013;14:1235-46.
- Wiseman-Hakes C, Murray B, Moineddin R, Rochon E, Cullen N, Gargaro J, Colantonio A. Evaluating the impact of treatment for sleep/wake disorders on recovery of cognition and communication in adults with chronic TBI. Brain Injury 2013;27:1364-76.
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science 2013;342:373-7.

