Key take home messages
- Clinically meaningful improvements are possible in chronic stroke patients
- The dose of rehabilitation treatment needs to be larger than currently delivered
- Rehabilitation is a complex intervention that cannot be reduced to a single element
Abstract
Somewhere between 50-80% of stroke survivors have upper limb symptoms after acute stroke1 and persistent difficulty in using the upper limb is a major contributor to ongoing physical disability.2 A commonly held view is that most recovery from stroke occurs over the first three to six months after which little improvement is possible, especially at the level of impairment.3-6 We argue that this may be a self-fulfilling prophecy resulting in lack of provision of potentially helpful rehabilitation.
What is the best way to promote upper limb recovery after stroke? Most studies of behavioural interventions have investigated forms of constraint induced movement therapy (CIMT),7,8 repetitive task training (RTT)9 or robotics,10 each of which focuses on increasing the activity of the affected limb. Kwakkel et al8 suggested that motor function, arm-hand activities and self-reported arm-hand functioning in daily life, improved immediately after CIMT and at long-term follow-up, but the comparison was often with usual care. It is worth noting that CIMT approaches were said to be more likely to be successful in promoting long term benefits if the protocol included shaping, massed practice and a behavioural transfer package, whereas simple forced use therapy was ineffective.8 RTT also has some evidence to support benefits over what is described as usual care, but the evidence for benefits over ‘matched therapy’ is less strong.9 The use of robotics can increase the number of movement repetitions, but has failed to produce clinically meaningful effects.10 Indeed, the recent RATULS study showed that compared with usual care, approximately 23 hours of robot-assisted training and matched dose ‘upper limb therapy’ did not improve upper limb function.11 Overall, it would appear that asking patients to make simple repetitions of movement, however meaningful the task, is relatively ineffective without some way of actively translating any improvements into activities of daily living. Simply increasing the number of repetitions does not appear to be effective,12 and this in itself should give us pause for thought.
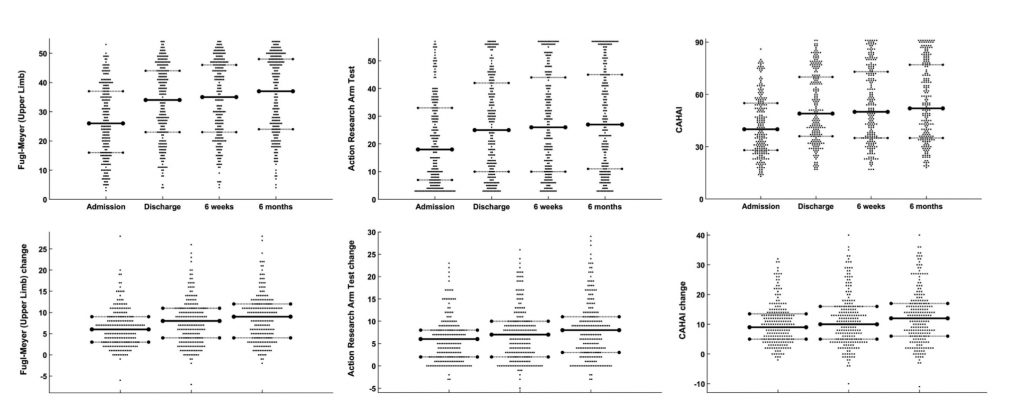
A few studies have tested more complex therapies incorporating a number of different elements. The ICARE study13 of upper limb treatment after stroke went beyond simple repetitions, using a structured, task-oriented motor training programme that was impairment focused, task specific, intense, engaging, collaborative, self-directed, and patient centred, starting about six weeks post-stroke. Outcomes were not improved by this approach, but on reflection it is likely that, as with many of the studies, the dose of 30 hours over ten weeks was too low (the usual care group received 11.2 hours over ten weeks). Despite scepticism that stroke patients would be able to ‘tolerate’ much higher doses,12 one study managed to deliver 300 hours of upper limb therapy to chronic stroke patients over twelve weeks and reported changes in measures of both impairment and activity that were far greater than those in lower dose studies,14 and in fact the findings of this study have recently been replicated by the same group.15 We recently reported the findings of the Queen Square Upper Limb (QSUL) Neurorehabilitation programme,16 a single centre clinical service that provides 90 hours of treatment focusing on the post-stroke upper limb. Most patients entering the programme were in the chronic stage (> 6 months post-stroke), but were able to complete the 90 hours of the programme, even though they exhibited a wide range of impairments and fatigue levels. Despite the time since stroke (median = 18 months) we observed (i) large clinically meaningful improvements in upper limb impairment and activity (of a magnitude similar to those reported by McCabe et al.), and importantly (ii) that these changes were maintained, or even improved upon, six months after treatment.
The first lesson to take from these studies is that post-stroke rehabilitation programmes and clinical trials are almost certainly under dosing patients. In future, clinical trials must investigate the effects of much higher doses than are currently being used. The second question to be raised is what are the key ‘active ingredients’ of an upper limb rehabilitation treatment? Whilst it is not clear what the optimal behavioural approach for promoting upper limb recovery should be, it is clear that simple protocol driven approaches have not led to large or sustained effects,17 both of which are necessary to produce a step change in stroke recovery. Successful post-stroke neurorehabilitation is likely to require a combination of complimentary approaches. If we accept this premise, then we are unlikely to determine the optimal combination of active ingredients simply by studying each approach in isolation, because the interactions between these elements will also have to be considered.
So how do we work out what the ‘active ingredients’ of upper limb rehabilitation are? A more sensible way forward is to look at interventions that have already demonstrated a high level of efficacy and then begin to work out their key components. Here, it is important to say that we need to start with treatments that have a high chance of achieving minimum clinically important differences (MCID) rather than small changes that might be statistically significant. Both McCabe et al14 and Daly et al,15 as well as the QSUL programme,16 produced large improvements on both impairment and activity limitation and both involved more complex treatment approaches, not restricted to one element. It is worth considering these in more detail.
- Analysis of movement and performance in activities of daily living. The initial assessment is crucial. The question, ‘why does this person’s hand and arm not work’ should never be answered with ‘because they have had a stroke’. There needs to be an appreciation of the range of potential contributory impairments (patterns of weakness, spasticity, loss of joint range, shoulder restriction and pain, sensory loss, apraxia, cognitive deficits, depression, apathy, fatigue etc.) because each of these becomes a therapeutic target. Our view is that without informed clinical reasoning based on the presence or absence of specific impairments, the correct treatment approach is unlikely to be selected.
- Identify and treat barriers. Avoid complications that will prevent participation in an active rehabilitation programme. We commonly see loss of passive joint range preventing people accessing finger or thumb movement, due to either spasticity or non-neural shortening. This can happen at most joints, but particularly in the hand. As well as increased finger flexion, be alert to loss of flexion at MCP joints which makes it difficult to shape the hand properly. Treatment involves splinting and optimal spasticity management. We also see pain and restriction of range in the shoulder. Restriction of external rotation in particular should raise the possibility of adhesive capsulitis. Despite the lack of a clear evidence base for treating post-stroke adhesive capsulitis, anecdotally we have had success with capsular hydrodilatation followed by physiotherapy.
- Preparation. Manual techniques are used to optimise and improve baseline at an impairment level, for example mobilising joints to improve range, lengthening and strengthening muscles to ensure they are at a biomechanical advantage to generate force, training sensory discrimination and improving postural control and balance.
- Reduction of impairment and re-education of quality and control of movement within activities of daily living. Individualised meaningful tasks are practiced repeatedly in order to facilitate task mastery with a focus on quality of movement. This is achieved through (i) adaptation of the task, e.g. decomposing tasks into individual components to be practiced; (ii) adaptation of the environment, e.g. fabrication of functional splints and adaptation of tools such as cutlery or screwdrivers, to enable integration of the affected hand in meaningful activities; (iii) assistance, e.g. de-weighting the arm to allow strengthening and training of movement quality and control through increased range.
- Coaching (involving instruction, supervision, reinforcement) was considered a key component of the QSUL programme, used throughout to embed new skills and knowledge into individual daily routines. Consequently, individuals increase participation and confidence in their desired goals, enhancing self-efficacy and motivation to sustain behavioural change beyond the end of the active treatment period.
- Sustaining change. Our view is that the approach described, delivered at a high dose is most likely to achieve clinically meaningful improvement together with improved self-efficacy and behaviour change that results in retention of gains or further improvement (something not routinely seen with many upper limb interventions that have been investigated).
Rehabilitation is often criticised for not following standardised approaches that lend themselves to investigation through clinical trials. However, when single elements are then studied in isolation the results are often not clinically meaningful and are not sustained.18,19 Looking at the difference between these approaches and those taken by McCabe et al14, Daly et al15 and QSUL16 should be informative, with a view to formally describing the key elements of a successful treatment. Whilst approaches at the activity and participation level will vary as they are tailored to an individual’s specific meaningful goals, the overall therapeutic approach taken towards specific impairments should be the same across all patients. Ideally, it should be possible to describe the principles of an optimal intervention using a format such as the TIDIER guidelines.18,19
There is a way to go before we can really say we understand both the treatment itself and the effects of the treatment on individuals. This will require careful assessment of both the ‘input’ (the nature of the behavioural intervention) and of the ‘output’ (the resulting behavioural change) at a level of fine-grained detail that is not currently achieved on a regular basis, for example using kinematic20 or neurophysiological21 assessment. In addition, this input-output relationship will be modulated by a number of patient characteristics, which could relate to behavioural characteristics (e.g. severity, presence of multiple impairments) or to biological characteristics (e.g. the nature and extent of brain damage, time since stroke, age, medication).
Overall, our experience suggests that much higher doses and intensity of upper limb neurorehabilitation can be delivered with beneficial effects. We have highlighted the need to consider the dose and the nature of the intervention as well as appropriate patient stratification in informing future clinical trial design.
References
- Lawrence ES et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32:1279–1284.
- Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21:357–364.
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–2186.
- Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:394–398.
- Sunderland A et al. Enhanced physical therapy for arm function after stroke: a one year follow up study. J. Neurol. Neurosurg. Psychiatr. 1994;57:856–858.
- Wade DT, Langton-Hewer R, Wood VA, Skilbeck CE, Ismail HM. The hemiplegic arm after stroke: measurement and recovery. J. Neurol. Neurosurg. Psychiatr. 1983;46:521–524 .
- Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database Syst Rev CD004433 (2015). doi:10.1002/14651858.CD004433.pub3
- Kwakkel G, Veerbeek J, van Wegen EEH, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015;14:224–234.
- French B et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev. 2016;11:CD006073.
- Veerbeek JM, Langbroek-Amersfoort AC, van Wegen, EEH, Meskers CGM, Kwakkel G. Effects of Robot-Assisted Therapy for the Upper Limb After Stroke. Neurorehabil Neural Repair. 2017;31: 107–121.
- Rodgers H et al. Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet (2019). doi:10.1016/S0140-6736(19)31055-4.
- Lang CE et al. Dose response of task-specific upper limb training in people at least 6 months poststroke: A phase II, single-blind, randomized, controlled trial. Ann. Neurol. 2016;80:342–354.
- Winstein CJ et al. Effect of a Task-Oriented Rehabilitation Program on Upper Extremity Recovery Following Motor Stroke: The ICARE Randomized Clinical Trial. JAMA. 2016;315:571–581.
- McCabe J, Monkiewicz M, Holcomb J, Pundik S, Daly JJ. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2015; 96:981–990.
- Daly JJ et al. Long-Dose Intensive Therapy Is Necessary for Strong, Clinically Significant, Upper Limb Functional Gains and Retained Gains in Severe/Moderate Chronic Stroke. Neurorehabil Neural Repair. 1545968319846120 (2019). doi:10.1177/1545968319846120.
- Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. J Neurol Neurosurg Psychiatry jnnp-2018-319954 (2019). doi:10.1136/jnnp-2018-319954
- Pollock A et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev. CD010820 (2014). doi:10.1002/14651858.CD010820.pub2
- Hoffmann TC et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348;g1687.
- Walker MF et al. Improving the Development, Monitoring and Reporting of Stroke Rehabilitation Research: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabil Neural Repair. 2017;31:877–884.
- Balasubramanian S, Colombo R, Sterpi I, Sanguineti V, Burdet E. Robotic assessment of upper limb motor function after stroke. Am J Phys Med Rehabil. 2012;91:S255-269.
- Cheung VCK et al. Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl. Acad. Sci. U.S.A. 2012;109:14652–14656.



One comment on “An expert opinion: upper limb rehabilitation after stroke”