Abstract
| Population based studies indicate that the incidence of SAH in the UK is around 10 per 100,000 of the population per annum with a median age at presentation of 61 years and a female preponderance (64%) [1]. Aneurysmal SAH is associated with smoking and hypertension. Early diagnosis and treatment is the key to reducing the high morbidity and mortality that is associated with this disorder [2]. |
Diagnosis and grading
The classical presentation of SAH is with a sudden severe headache accompanied by vomiting, photophobia and neck stiffness. Other less obvious cases of sudden severe headache with resolution over 24-48 hours may lead to a missed or delayed diagnosis and therefore a high index of clinical suspicion is required when taking a history. Some patients present in coma.
All patients with a suspected SAH require an urgent CT brain scan to demonstrate haemorrhage. The sensitivity of this investigation exceeds 95% if performed within 24h of the ictus. CT angiography is often utilised in specialised units to help delineate any aneurysms at this stage and this may even replace invasive cerebral angiography. If the initial CT scan is normal a lumbar puncture should be performed without delay, since uniformly blood stained CSF supports a diagnosis of a Sylvian Fissure Haematomas and warrants further investigation regardless of the presence of xanthochromia.
Patients with a SAH can be graded according to their clinical status [3]. Poor grade patients (not obeying commands) require airway protection and are best ventilated for transfer to a regional centre.
Early Management (Figure 1)
The principal causes of deterioration and death in patients with a SAH are severe primary haemorrhage, rebleed, delayed cerebral ischaemia, hydrocephalus and general medical complications and so the early management is directed at minimising the overall risk of such complications.
A severe haemorrhage contributes to a cycle of elevated intracranial pressure, cerebral ischaemia and catastrophic cerebral oedema. Respiratory and circulatory support may improve the situation. Patients with clinical signs of dehydration are at an increased risk of developing delayed cerebral ischaemia and so early fluid administration of saline alternating with colloid supplemented by oral intake should be established in good grade patients [4]. Central venous pressure monitoring to titrate fluid administration in poor grade patients is essential.
Nimodipine is a selective calcium channel antagonist that should be administered to all patients. It is proven to reduce the incidence of a poor outcome from delayed cerebral ischaemia [5]. Anticonvulsants are only recommended if seizure activity has occurred.
Referral
All patients except those with fixed dilated pupils from a catastrophic presentation haemorrhage should be
urgently referred to the regional neurosurgical service. Patients who do not obey commands should be ventilated for transfer.
Specialist Management (Figure 1)
Specialists need to recognise and treat hydrocephalus and cerebral ischaemia in addition to providing definitive
aneurysm treatment. The insertion of an external ventricular drainage in patients who do not obey commands
where the CT scan reveals ventricular dilatation can improve the clinical grade. Patients who obey commands do not require CSF drainage unless their neurological state deteriorates. External ventricular drains should be tunnelled posteriorly to minimise encroachment upon any subsequent craniotomy site.
[Read more ACNR neurosurgery articles]
Four-vessel cerebral angiography is performed in good grade patients within 24-48 hours of transfer. Ventilated poor grade patients are assessed neurologically after treatment of hydrocephalus and stabilisation on the critical care unit. If they localise to pain, or better, after cessation of sedation, angiography is undertaken. Whilst the CT scan appearances help to determine the likely location of the culprit aneurysm the entire intracranial circulation is inspected to determine the presence of multiple aneurysms (which occur in around 20% of cases). The advent of 3-D reconstruction technology has improved angiographic interpretation and aids both endovascular and surgical approaches.
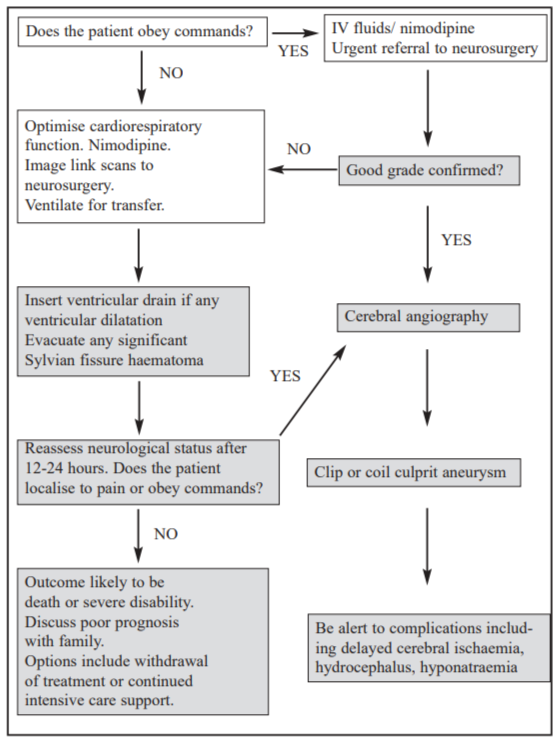
Endovascular management
Over the past decade endovascular techniques have become the treatment of choice for ruptured posterior fossa aneurysms. Publication of the interim results of the International Subarachnoid Aneurysm Trial (ISAT) has supported the development of aneurysm coiling for anterior circulation aneurysms [6]. Although the long term durability of coils has not yet been adequately determined, the absolute difference of 6.9% in poor outcome at 12 months has led to a large increase in the number of coiling procedures undertaken nationwide. Coiling is performed under general anaesthetic and close liaison between radiologist and surgeon is recommended to discuss optimal management. A sub-optimal coiling may be appropriate in elderly and/or poor grade patients whereas significant aneurysm remnants are less acceptable in younger, good grade patients (Figure 2). If coiling is not appropriate open surgery is undertaken provided the patient localises to command.
Surgical Management
Aneurysm surgery is usually performed within 72 hours of the ictus to minimise the re-bleed risk and enable aggressive treatment of any subsequent delayed ischaemic deficit.
Technical Aspects of Surgery
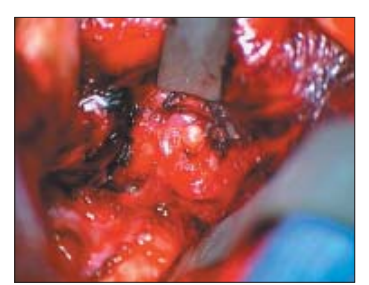
Most anterior aneurysms are approached through an ipsilateral pterional craniotomy. The scalp is opened as a single layer and a Z-shaped incision is made in the temporalis fascia and frontal periosteum permitting exposure of the pterion. A single burrhole in the posterior temporal region is incorporated into a low pterional craniotomy. A cresenteric dura flap is opened in a dry operative field with a halo retractor system in place. The brain can appear very swollen at this stage. However, placement of a subfrontal 8mm retractor with microscope assistance enables visualisation of the optic nerve. The chiasmatic, carotid and lamina terminalis cisterns are then opened and brain decompression is achieved. The temporal bridging veins are divided before placing a 6mm retractor on the temporal lobe to expose the Sylvian fissure. This is opened to provide direct access to the internal carotid artery and the bifurcation. Sub-pial resection of brain adjacent to the middle or anterior cerebral arteries minimises traction on the subarachnoid vessels as the aneurysm complex is encountered (Figure 3). Great care is taken to identify the anatomy of the aneurysm. With anterior communicating artery aneurysms the contralateral A1 and A2 vessels can usually be identified by inspection across the chiasm. With large MCA aneurysms the sac may need to be retracted to identify the more medially placed M2 efferent vessels. Time spent dissecting around the aneurysm neck minimises the risk of tearing the arachnoid causing an intraoperative rupture during clip placement. Cauterisation of the aneurysm sac may enable moulding of the aneurysm permitting better placement of a clip. Clip placement is frequently more safely achieved with a short period of parent vessel temporary clipping to reduce the tension in the aneurysm sac. Clip blades should be placed parallel to the parent vessel to prevent kinking or occlusion of parent and distal vessels. The dome of the clipped aneurysm is punctured to ensure that it has been satisfactorily secured. If an intraoperative rupture occurs, aspirate blood without increasing the size of the rent in the aneurysm. Often a small bleed will cease with suction, irrigation, precise coagulation and pressure. Fenestrated and encircling clips can be used to effectively rescue a tense situation should the rent be near the aneurysm neck. Once the aneurysm is secure the dura is closed, the bone flap replaced, the temporalis reconstructed and the scalp closed.
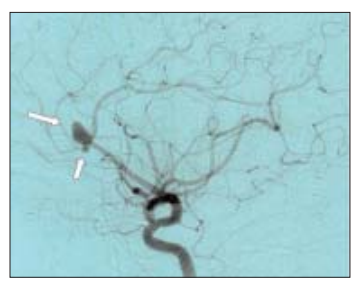
Sylvian fissure haematomas
Patients presenting with a Sylvian fissure haematoma, depressed consciousness and a fixed dilated pupil require urgent intervention if any chance of a reasonable outcome is to be offered. A dilemma occurs between delaying treatment for investigation and undertaking surgery without knowledge of the underlying pathology. If a CT angiogram can be performed at presentation, valuable information can be gained without undue delay but in the emergency situation formal cerebral angiography is not warranted. The surgical tactics are determined by the experience of the operator. In non-expert hands partial haematoma evacuation with removal of the bone flap is reasonable pending a delayed definitive procedure to secure the aneurysm. In expert hands an extended pterional flap and subfrontal exposure of the optic nerve is recommended. The aneurysm (which is usually a laterally projecting middle cerebral aneurysm or an internal carotid bifurcation aneurysm) can then be dissected and a final clip placed usually under the protection of a proximal temporary clip.
Post-clipping/coiling management
The principal causes of post-treatment deterioration are distal vessel compromise as a result of treatment, delayed ischaemic deficit and hydrocephalus. The former is minimised by careful attention during the procedure. Aspirin or heparin may be beneficial to minimise the risk of an early intraluminal thrombosis in patients who have undergone endovascular treatment where the coil projects into the parent vessel.
Vasospasm should be managed prophylactically and therapeutically. A target systolic blood pressure of around 160 mmHg and CVP of 8-10 cm are achieved using fluids and inotropes as required. Should clinical features of vasospasm become evident the blood pressure is further augmented to evaluate whether improvement occurs. Careful monitoring of electrolytes is mandatory at this stage. Hyponatraemia should not be managed with fluid restriction since this is associated with a high incidence of cerebral infarction [4]. The value of angioplasty or papaverine injection in patients with symptomatic vasospasm requires further evaluation in the setting of a randomised controlled clinical trial. Hydrocephalus is treated with ventricular or lumbar drainage of CSF. A shunt can transform the outcome for a small number of patients.
Prognosis
A 1992-96 population based study in the UK showed that 16% of patients with a SAH died without receiving medical attention – presumably due to a catastrophic sudden presentation. A further 5% of patients died within 24h of ictus. By day 3 the case fatality rate was 30%. [1] A recent National SAH audit indicated that about 30% of catastrophic SAH cases suffer from rebleeds, about 30% develop cerebral ischaemia and about 30% deteriorate from hydrocephalus. By day 30 the case fatality rate increased steadily to 44%, probably due to the high morbidity of delayed cerebral ischaemia and the prevalence of medical problems in patients with SAH. [1] Natural history studies indicate that without treatment approximately 20% of aneurysms will re-rupture within 2 weeks of the first bleed [7].
Non-specific symptoms associated with SAH include headaches, lethargy, poor concentration and short-term memory deficits. Such cognitive problems can be the source of major disability [8].
The prognostic factors predictive of a poor outcome are increasing age, poor grade, presence of extensive intraventricular haemorrhage and the development of delayed cerebral ischaemia. In good grade patients a favourable outcome is achieved in 90% of patients with death and severe disability occurring in a minority [9]. However, the prognosis in poor grade patients is generally poor with only a minority achieving a favourable outcome [10].
Summary
The management of SAH is now multidisciplinary from the outset. Neurosurgeons, radiologists and intensivists
all provide significant contributions to early patient care. Whilst coil technology and intracranial angioplasty
evolve, surgery becomes less commonplace. However, surgery for intracranial aneurysms provides a major challenge that requires skill, dedication and attention to detail. The young ambitious neurosurgeon will find great satisfaction from aspiring to treat this cohort of patients.
References
- Rinkel GJE, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture on intracranial aneurysms. A systematic review. Stroke 1998;29:251-6.https://doi.org/10.1161/01.STR.29.1.251
- Pobereskin LH. Incidence and outcome of subarachnoid haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry 2001;70:340-3. https://doi.org/10.1136/jnnp.70.3.340
- Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg 2000;93:379-87. https://doi.org/10.3171/jns.2000.93.3.0379
- The International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms – risk of rupture and risks of surgical intervention. New England J Med 1998;339:1725-33. https://doi.org/10.1056/NEJM199812103392401
- International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103-10. https://doi.org/10.1016/S0140-6736(03)13860-3
- Kassell NF, Torner JC, Haley ECJ, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg 1990;73:18-36. https://doi.org/10.3171/jns.1990.73.1.0018
- Molyneux AJ, Kerr RSC, Yu L-M, Clarke M, Yarnold JA, Sandercock P. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups and aneurysm occlusion. Lancet 2005;366:809-17. https://doi.org/10.1016/S0140-6736(05)67214-5
- Vindlacheruvu RR, Mendelow AD, Mitchell P. Risk-benefit analysis of the treatment of unruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry 2005;76:234-9. https://doi.org/10.1136/jnnp.2003.031930
- White PM, Wardlaw JM. Unruptured intracranial aneurysms. Detection and management. J Neuroradiol 2003;30:336-50.
- Teasdale GM, Wardlaw JM, White PM, Murray G, Teasdale EM, Easton V. The familial risk of subarachnoid haemorrhage. Brain 2005;128:1677-85. https://doi.org/10.1093/brain/awh497
