
Reader in Clinical Neurology, UCL Institute of Neurology, National Hospital for Neurology and Neurosurgery, Queen Square, WC1N 3BG.
In stroke medicine, some of the most worrying decisions clinicians have to make are related to balancing the risk of future occlusive cerebral and systemic ischaemic events (for which antithrombotic drugs are likely to help) and the risk of intracerebral bleeding (which may be caused or aggravated by antithrombotic drugs). Small vessel disease processes that cause most cerebral haemorrhages can also lead to ischaemic stroke (even in the same patient). The stakes here are high because antithrombotic-related intracerebral haemorrhage is often devastating or fatal. In the latest article in ACNR’s Stroke series we are therefore delighted to have a timely, authoritative and thought-provoking article on the challenges in managing antithrombotic drug treatment after an intracerebral haemorrhage. In this very clear and concise review, Rustam Al-Shahi Salman and Simon Bell describe the limits of available evidence to guide us, and emphasise the importance of ongoing randomised trials to help resolve this most challenging of modern stroke medicine dilemmas.
Summary
- Stroke due to intracerebral haemorrhage (ICH) causes more lost disability-adjusted-life-years than ischaemic stroke
- Acute stroke unit care and blood pressure reduction for secondary prevention of stroke improve outcome for ICH survivors
- When patients suffer an ICH, almost half are being treated with an antithrombotic drug
- But should survivors of antithrombotic-associated ICH restart or avoid antithrombotic drugs?
- Join the on-going collaboration of 114 hospitals in the UK helping to answer this question in the REstart or STop Antithrombotics Randomised Trial (RESTART, http://www.RESTARTtrial.org, ISRCTN71907627)
Stroke due to intracerebral haemorrhage (ICH) is a burden
Stroke due to non-traumatic intracerebral haemorrhage (ICH) affects roughly 15,000 adults in the UK and ~2 million adults elsewhere in the world each year.1 Despite ICH accounting for only 10-15% of all strokes, ICH causes more lost disability-adjusted-life-years than ischaemic stroke.1 ICH accounts for an even larger proportion of strokes in low-middle income countries,2 where hypertension is particularly prevalent.3
First, the good news…
Stroke due to ICH is treatable
Acute stroke unit care is just as much of a life-saving management strategy for patients with ICH as for their counterparts with acute ischaemic stroke.4 Furthermore, acute blood pressure lowering within six hours of ICH onset is safe, and seems to reduce disability (but not death).5 The recently updated European Stroke Organisation (ESO) ICH guidelines strongly recommended acute stroke unit care for patients with ICH and made a weak recommendation for acute blood pressure lowering. The writing group did not find evidence from randomised trials to support other interventions for acute ICH, such as surgical evacuation.6
Stroke due to ICH is preventable
The PROGRESS randomised trial involving 6,105 patients with prior ischaemic stroke, ICH or transient ischaemic attack has shown that reducing blood pressure is the most important intervention for secondary prevention after ICH.7 PROGRESS showed that over a mean follow-up of four years, the overall relative risk of recurrent stroke was reduced by 28% in the group treated with an ACE inhibitor (perindopril) with/without a diuretic (indapamide) compared to the placebo group.7 The benefits of blood pressure reduction were greatest for patients with ICH,8 regardless of the presumed underlying small vessel disease and whether or not the patient was ‘hypertensive’,9 prompting the ESO ICH guidelines to strongly recommend reducing blood pressure after ICH.6
But what should be done about antithrombotic drugs after ICH?
Patients with ICH often have co-morbid ischaemic diseases and other ‘vascular risk factors’ in addition to hypertension. Because evidence in recent decades has shown the benefits of antithrombotic (i.e. antiplatelet and anticoagulant) drugs for the prevention of major cardiovascular events,10,11 it is unsurprising that the use of antithrombotic drugs at the time of ICH has risen to 40-50% during the same timespan.12,13
Given ICH patients’ co-morbidities, they are at considerable risk of further major vascular events; overall, ischaemic events appear to be at least as frequent as recurrent ICH.14 Ischaemic events may be even more common in ICH survivors with a past history of ischaemic events, but there are few data about this sub-group of interest.
So this raises the not infrequent clinical dilemma of whether to restart or avoid antithrombotic drugs in survivors of ICH who have a clear indication for these drugs.15
Unfortunately, there are no randomised trials addressing this uncertainty about secondary prevention treatment.6,16 It is therefore unsurprising that there is variation in clinical practice when it comes to restarting these drugs, which does not appear to be explained by any patient characteristics.17
Antiplatelet drugs
Three published observational studies describe the outcomes associated with restarting or avoiding antiplatelet drugs after ICH (Table 1).18-20 One study found a two-fold reduction in all major vascular events among patients who restarted aspirin after any ICH.19 None of the studies found an increase in the risk of recurrent ICH associated with restarting aspirin in univariate analyses. However, one of these studies of 104 adults with lobar ICH identified 29 recurrent ICHs during a median follow-up of almost three years.18 In a multivariable analysis of this small cohort, there was an association between aspirin use after ICH and recurrent ICH (adjusted hazard ratio 3.95, 95%CI 1.6-8.3), which was possibly explained by microbleeds.18
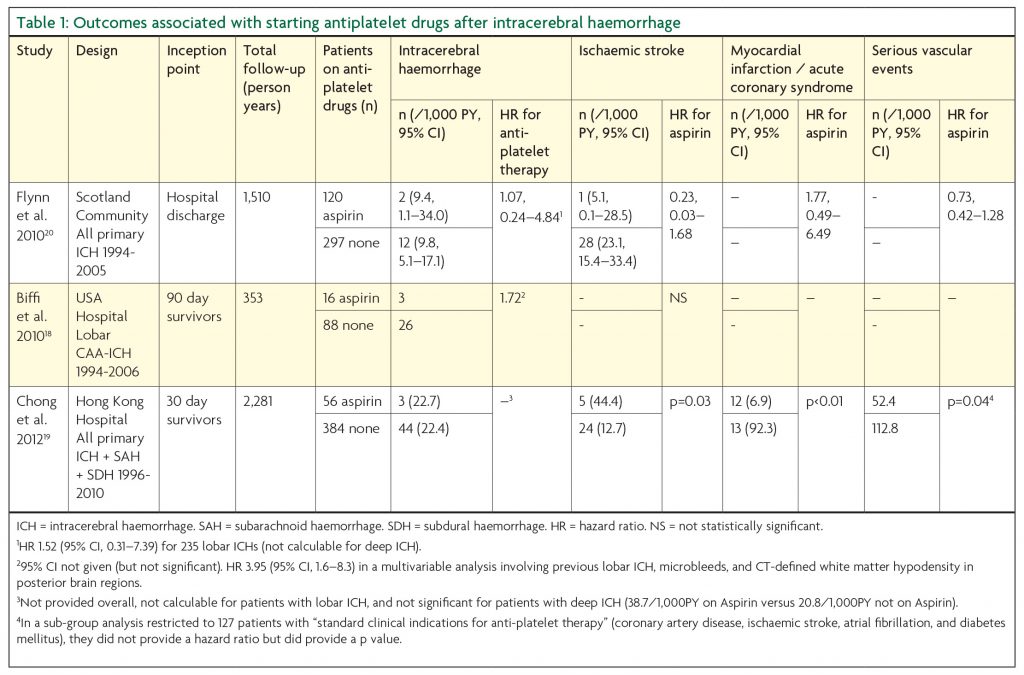
The logical response to this uncertainty, in the light of variation in practice, the lack of dramatic effects in observational studies, and the possibility of confounding by indication in observational studies, is a randomised trial. The REstart or STop Antithrombotics Randomised Trial (RESTART, http://www.RESTARTtrial.org, ISRCTN71907627, Figure 1) started to address this uncertainty in May 2013, and has recruited 168 participants at 114 UK hospitals at the time of writing. RESTART ultimately seeks to determine whether restarting antiplatelet drugs results in a beneficial reduction in all major vascular events. The trial is currently powered to address the safety of doing so. Given an annual risk of recurrent symptomatic ICH of about 1.8 to 7.4% per annum14 and observational studies showing a 1- to 4-fold relative increase in the risk of recurrent ICH on antiplatelet drugs (Table 1), if RESTART recruits 720 patients it will have excellent power (after all participants have been followed for at least two years) to detect a doubling of the rate of ICH if the true rate is 4.5% per annum, or 93% power at the 5% significance level to detect a 4-fold increase in risk of recurrent ICH if the annual risk is only 1%.
Stroke-friendly UK Neurologists are encouraged to join the RESTART collaboration by contacting RESTART.trial@ed.ac.uk to help us resolve this clinical dilemma.
Anticoagulant drugs
At least twelve observational studies have compared the outcome of restarting or avoiding oral anticoagulant drugs after intracranial haemorrhage, but only four of these studies were sufficiently described and large enough to inform the dilemma about whether or not to restart oral anticoagulation (Table 2).21-24 These four studies were of adults with either purely intracerebral24 or various types of intracranial haemorrhage21-23 and a variety of indications for oral anticoagulation (although analyses were restricted to patients with atrial fibrillation in two studies23,24). Although the studies chose different inceptions points, they all described outcomes over approximately one year of follow-up. However, the studies described a variety of different outcome events, so only the most frequently reported are shown in Table 2, but the frequencies of these events vary probably because of differences in study design. Nevertheless, these studies described promising associations between oral anticoagulants and reductions in: death of any cause,23,24 all ischaemic events,23,24 ischaemic stroke,21 and ischaemic and haemorrhagic events combined.23 Three studies described non-significant associations between oral anticoagulants and haemorrhagic events22-24 and one study found a significant increase in the risk of haemorrhagic events on oral anticoagulants.21
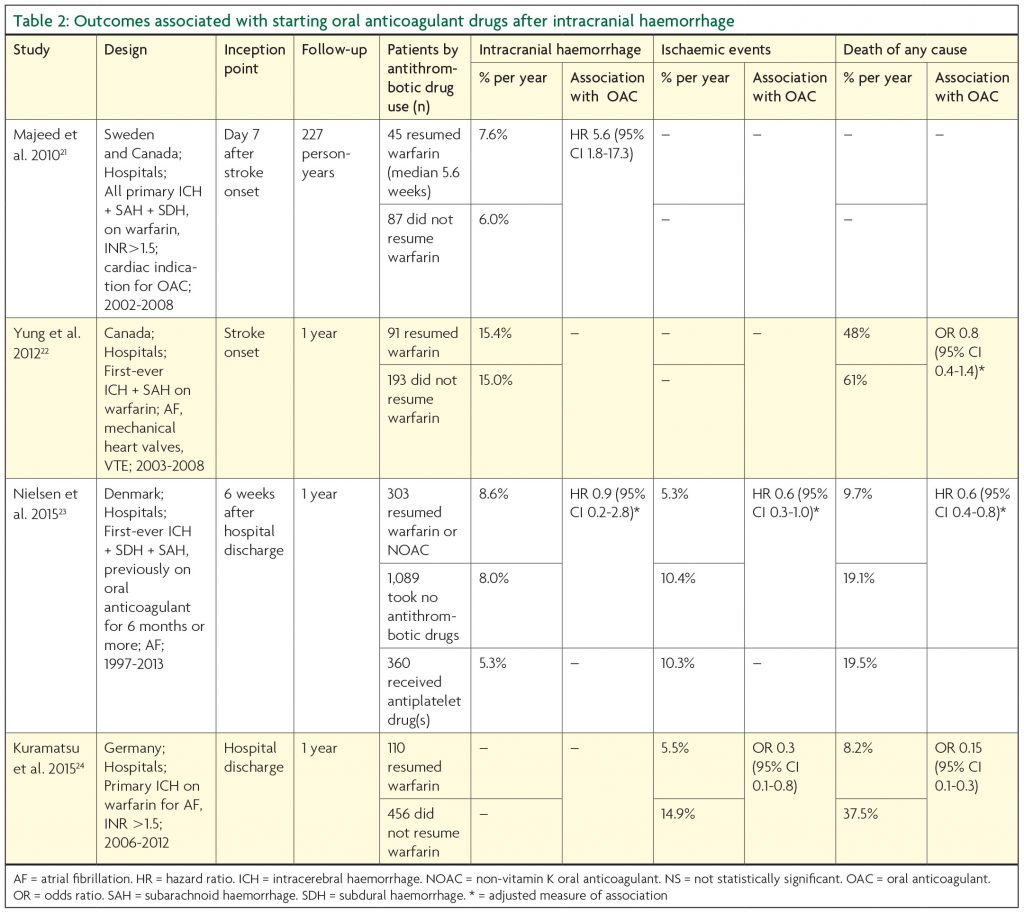
Again, the associations seen in these observational studies and the possibility of confounding by indication mean that a randomised trial is warranted to investigate whether resuming an oral anticoagulant results in a beneficial reduction in all major vascular events. The non-vitamin K oral anticoagulants are particularly attractive for such a trial, because of their lower risk of intracranial haemorrhage in comparison to warfarin.25 Stroke-friendly UK Neurologists are encouraged to contact RESTART.trial@ed.ac.uk if they would be interested in joining our efforts to obtain funding for this trial.
Conclusion
Double-edged swords generate many of the contemporary dilemmas in stroke medicine: cerebral small vessel diseases may manifest with ischaemia or haemorrhage; biomarkers such as brain microbleeds are associated with the occurrence of both haemorrhagic and ischaemic clinical outcomes; and antithrombotic drugs cause beneficial reductions in ischaemic clinical outcomes at the expense of an increase in the risk of haemorrhagic clinical outcomes.
These “first world” dilemmas are becoming increasingly frequent as survival after stroke improves and society ages, and will become a problem elsewhere in the world as epidemiological transitions occur in low-middle income countries. However, treatment uncertainties arising from these dilemmas require resolution in large randomised trials, with embedded advanced imaging sub-studies to explore the potential of stratified medicine approaches.
The only hope for us to resolve dilemmas such as these is large-scale collaboration within ‘learning healthcare systems’ that embed clinical research in the routine of everyday clinical practice.26,27
References
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. The Lancet Global Health 2013;1:e259-81.
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. The Lancet Neurology 2009;8:355-69.
- Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013;310:959-68.
- Langhorne P, Fearon P, Ronning OM, et al. Stroke unit care benefits patients with intracerebral hemorrhage: systematic review and meta-analysis. Stroke 2013;44:3044-9.
- Tsivgoulis G, Katsanos AH, Butcher KS, Boviatsis E, Triantafyllou N, Rizos I, Alexandrov AV. Intensive blood pressure reduction in acute intracerebral hemorrhage: a meta-analysis. Neurology 2014;83(17):1523-9.
- Steiner T, Al-Shahi Salman R, Beer R, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. International Journal of Stroke 2014;9:840-55.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033-41.
- Chapman N, Huxley R, Anderson C, et al. Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS Trial. Stroke 2004;35:116-21.
- Arima H, Tzourio C, Anderson C, et al. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke 2010;41:394-6.
- Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60.
- Alberts MJ, Eikelboom JW, Hankey GJ. Antithrombotic therapy for stroke prevention in non-valvular atrial fibrillation. The Lancet Neurology 2012;11:1066-81.
- Lovelock CE, Molyneux AJ, Rothwell PM, Oxford Vascular S. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. The Lancet Neurology 2007;6:487-93.
- Bejot Y, Cordonnier C, Durier J, Aboa-Eboule C, Rouaud O, Giroud M. Intracerebral haemorrhage profiles are changing: results from the Dijon population-based study. Brain 2013;136:658-64.
- Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. Journal of neurology, neurosurgery, and psychiatry 2014;85:660-7.
- Selim MH, Molina CA. Elderly and forgetful: is aspirin safe for you? Stroke 2014;45:3153-4.
- Flynn RW, MacDonald TM, Murray GD, Doney AS. Systematic review of observational research studying the long-term use of antithrombotic medicines following intracerebral hemorrhage. Cardiovascular therapeutics 2010;28:177-84.
- Pasquini M, Charidimou A, van Asch CJ, et al. Variation in restarting antithrombotic drugs at hospital discharge after intracerebral hemorrhage. Stroke 2014;45:2643-8.
- Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010;75:693-8.
- Chong BH, Chan KH, Pong V, et al. Use of aspirin in Chinese after recovery from primary intracranial haemorrhage. Thrombosis and haemostasis 2012;107:241-7.
- Flynn RW, MacDonald TM, Murray GD, MacWalter RS, Doney AS. Prescribing antiplatelet medicine and subsequent events after intracerebral hemorrhage. Stroke 2010;41:2606-11.
- Majeed A, Kim YK, Roberts RS, Holmstrom M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010;41:2860-6.
- Yung D, Kapral MK, Asllani E, Fang J, Lee DS. Investigators of the Registry of the Canadian Stroke N. Reinitiation of anticoagulation after warfarin-associated intracranial hemorrhage and mortality risk: the Best Practice for Reinitiating Anticoagulation Therapy After Intracranial Bleeding (BRAIN) study. The Canadian journal of cardiology 2012;28:33-9.
- Nielsen PB, Larsen TB, Skjoth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY. Restarting Anticoagulant Treatment After Intracranial Haemorrhage in Patients With Atrial Fibrillation and the Impact on Recurrent Stroke, Mortality and Bleeding: A Nationwide Cohort Study. Circulation Published online before print June 9, 2015, doi: 10.1161/CIRCULATIONAHA.115.015735.
- Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015;313:824-36.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62.
- Faden RR, Beauchamp TL, Kass NE. Informed consent, comparative effectiveness, and learning health care. The New England journal of medicine 2014;370:766-8.
- Al-Shahi Salman R, Beller E, Kagan J, et al. Increasing value and reducing waste in biomedical research regulation and management. Lancet 2014;383:176-85.

