Abstract
There is widespread consensus that symptomatic unruptured aneurysms carry a substantial risk of rupture and require treatment. In contrast the management of a patient with an asymptomatic unruptured intracranial aneurysm (AUIA) remains controversial. If all AUIAs could be successfully obliterated with minimal morbidity and mortality the catastrophic consequences of a subarachnoid haemorrhage could be safely averted. However, the attendant risks of treatment may outweigh the potential benefits of aneurysm exclusion. Therefore it is important to appraise the natural history of these lesions if left untreated and balance this against the risks of treatment when formulating an evidence-based management plan.
Epidemiology
Epidemiological data can help estimate the risk of AUIA rupture. Post mortem and angiographic studies report that AUIAs are found in approximately 1-6% of the population. [1] The variability in incidence may be attributed to geographic and age profile differences in the populations studied in addition to case selection, technical and observer factors. In a systematic review Rinkel et al. conclude that the prevalence of AUIAs in adults without specific risk factors is 2.3%. [1]
A population based study with stringent case ascertainment indicates that the incidence of aneurysmal subarachnoid haemorrhage is 7.4/100 000 men and 11.9/100 000 women in South West England, with an overall rate of 9.7/100 000. [2] The relatively high prevalence of AUIA and low incidence of subarachnoid haemorrhage indicates that most AUIA do not rupture.
If all aneurysmal subarachnoid haemorrhages (1/10000 per annum) are considered to originate from AUIAs and the prevalence of AUIA is considered to be 2.3%, the risk of rupture can be estimated as 0.4% per annum. This may be an over estimate due to the unknown number of cases of rapid de novo aneurysm formation, expansion and rupture with no significant time spent in an AUIA phase. In addition, risk factors (smoking, hypertension, polycystic kidney disease) will increase the rupture rate in some patients. Assuming that virtually all AUIAs and SAH cases occur in the 60% of the population over the age of 30 years, the incidence of SAH in adults can be approximated to 1 in 6000 while the prevalence of AUIA is 1 in 26 in this population. This again infers that less than 0.5% of AUIA will rupture per annum.
Natural History Studies
It is difficult to study the natural history of AUIAs both due to the longevity required, cases lost to follow-up and the fact that cohort studies may carry significant bias due to the exclusion of patients selectively treated and not recruited for follow-up. In such studies 2 groups of patients are characterised; those with an incidental AUIA and cases with an additional AUIA. The latter group have previously had aneurysmal SAH from a separately treated culprit aneurysm. Valuable follow-up data in 142 patients with 181 unruptured intracranial aneurysms with prolonged follow-up has been reported from Scandinavia (mean follow up 19.7 years, range 0.8 – 38.9 years, 109 cases studied for at least 10 years). [3] The majority (n = 131) of these cases were additional aneurysm patients. Of these, 30 patients experienced a further SAH during follow-up. This was calculated to represent an annual rupture incidence of 1.3%. The risk factors for haemorrhage were age (inverse relationship), increasing aneurysm size and smoking. This study did not provide any robust information about the rupture risk from incidental AUIAs.
The International Study of Unruptured Intracranial Aneurysms (ISUIA) is a 53-centre study that has provided natural history data. [4,5] The 1449 retrospectively studied patients presenting between 1970 and 1991 showed that the incidence of rupture in patients with incidental anterior circulation aneurysms <10 mm in diameter was 0.05% per annum. In patients with similar size aneurysms and a previous SAH the rupture risk was 0.5% per annum. The rupture rates for aneurysms between 10- 15mm approached 1% for both groups. [4] Whilst it may seem that over 3 decades the risk would be around 30%, mathematical probability indicates that it is lower at around 22%. These retrospective findings indicate a much lower rupture rate than previously reported. Although inclusion bias has been cited as a possible reason for these results, many clinicians have adopted a more conservative approach in response to this evidence.
[Read more ACNR neurosurgery articles]
The prospective component of the ISUIA study with recruitment between 1991-1998 followed up 1692 patients who had conservative management of incidental (1077 cases) or additional (615 cases) AUIAs for an average of 4.1 years. [5] Interestingly the incidental aneurysms were generally larger than the additional lesions. Of the 51 patients (3%) that sustained a SAH during follow-up, 41 had incidental aneurysms with no history of previous SAH (3.8%). Only 10 (1.6%) cases in the additional aneurysm group sustained a SAH. The large number of very small aneurysms in this group (80% compared to 50% in the incidental aneurysm group) probably explains the lower bleed rate. Whilst a previous SAH has been shown to be associated with a higher risk of AUIA rupture in the retrospective cohort the small numbers of prospectively observed bleeds in this group does not add credence to this conclusion. This study also reported several other notable findings related to aneurysm size and location.
1. Incidental aneurysms less than 7 mm in diameter very rarely rupture (n=2). Both ruptured aneurysms in this size range were posterior communicating artery aneurysms. Since no other 2–7 mm incidental anterior circulation aneurysms ruptured, posterior communicating artery aneurysms were speculated to carry a higher risk of bleeding and were subsequently analysed with posterior circulation aneurysms. Seven of the 10 ruptured additional aneurysms were in the 2-7 mm size bracket.
2. Larger incidental aneurysms are more likely to bleed. This conclusion was largely based upon the findings in patients with incidental aneurysms. The numbers of ruptures in the additional aneurysm group were too small to comment upon the importance of size.
3. Aneurysms in the posterior circulation had a higher rate of rupture. The 5-year rupture rates are summarised in Table 1. In essence the annual risk of a bleed was less than 1% for aneurysms up to 12 mm diameter, only increasing to around 3% for lesions 13-24 mm. The rupture rate for giant aneurysms was around 10% per annum. It is important to recognise that the ISUIA study was not a randomised-controlled trial and that many patients eligible for conservative observation were selected for surgical intervention or endovascular therapy. In addition, the use of inclusion criteria and the unknown recruitment rate question the applicability of the findings to the wider population. Despite these caveats the study does provide the best natural history data available. Future reports from ISUIA with longer follow-up will enhance the quality of the data.
Operative and endovascular treatment risks
The reporting of procedural risks is imperfect due to publication bias and criticisms of study design. Whilst the International Cooperative Study on aneurysmal SAH compared surgical treatment with overall management outcome in a cohort of 3500 cases such information is not directly applicable to the AUIA population. [6] Similarly the ISAT trial was targeted at patients treated after a subarachnoid haemorrhage. [7] The most robust information again arises from the prospective cohort of the ISUIA study where outcome was recorded in 1917 cases undergoing aneurysm clipping and 451 cases treated with endovascular coiling recruited between 1991 and 1998. [5] Risk factors for a poor outcome were posterior fossa aneurysm location, size greater than 12mm and (for surgical patients only) age. A considerable proportion of endovascular treatments only achieved partial occlusion; the long-term durability of this treatment remains under scrutiny. Overall, poor outcomes were reported in around 5-6% of clipped small and medium sized anterior circulation aneurysms in patients less than 50 years of age. Age over 50 years doubled the frequency of poor outcomes following clipping of small aneurysms. The poor outcome rate increased to around 25% for patients over 50 years with medium sized aneurysms. Morbidity from posterior fossa aneurysm surgery was even greater. Endovascular coiling of small and medium sized anterior circulation aneurysms carried a 7-8% risk of a poor outcome in patients less than 50 years old. Although these results suggest that small aneurysms are best treated with clipping it should be remembered that the patients were selected and not randomised to different treatment modalities. Giant aneurysms were rare but the risks of a poor outcome with endovascular treatment were around 15% with wide confidence limits.
Risk assessment
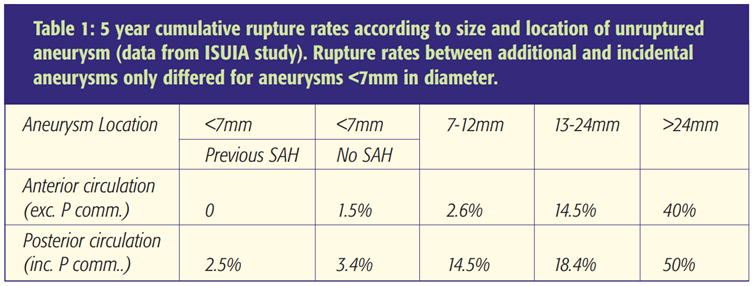
ISUIA and Juvela’s cohort study have provided an estimate of aneurysm rupture rate over a period of time. [3,4,5] ISUIA has also provided data on treatment outcomes in patients undergoing prophylactic aneurysm treatment. For each individual the risk of treatment needs to be balanced against the risk of rupture. Using the ISUIA data and predicted life expectancy derived from the 2001 UK census, Mitchell’s group plotted outcome predictive curves where “life years saved or lost by aneurysm repair” was plotted against age at time of treatment (see Figures 1 and 2) [8] Inspection of the curves enables the clinician to ascertain the relative risks of treatment versus the saving of life years. For example, life years are saved by endovascular treatment of incidental anterior circulation aneurysms in the 13-24 mm range for patients under the age of 65 years. This model is based on the assumption that aneurysm occlusion provides robust protection from rebleeds. It does not address the cost-benefit ratio of treatment.
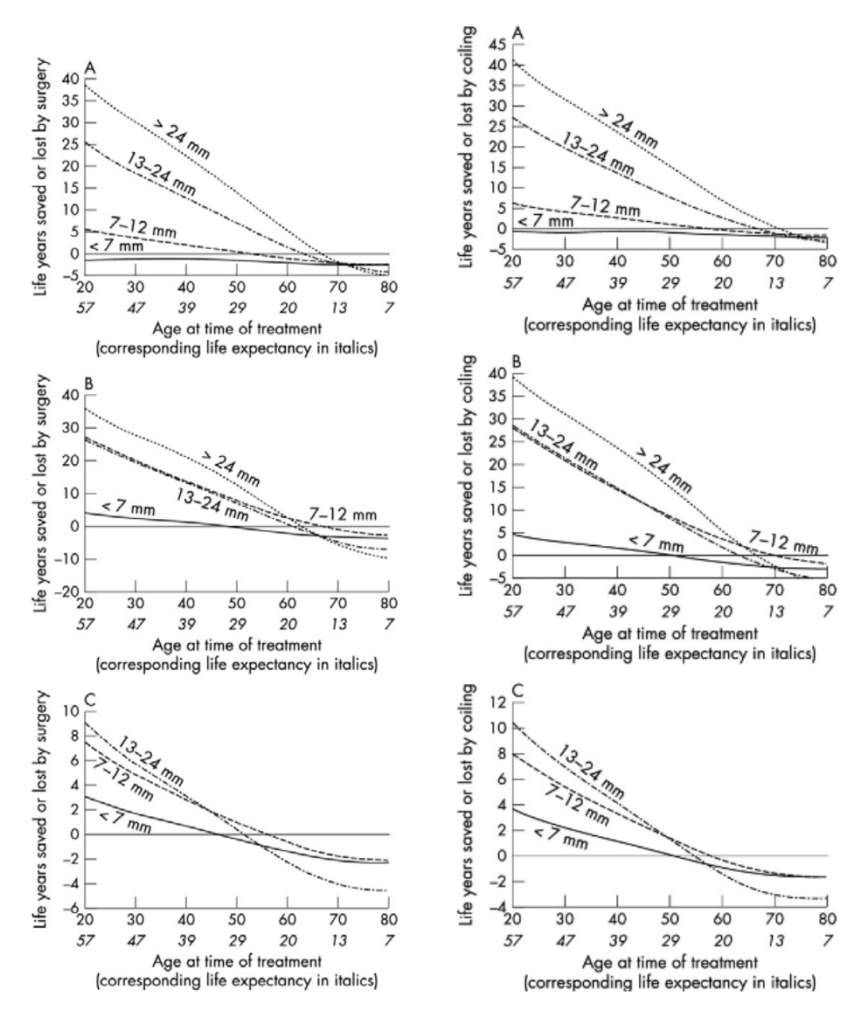
*Figure 2 (right column): Expected life years lost or gained by treatment against patient age at the time of treatment for endovascular treatment of unruptured aneurysms in four size ranges. (A) Incidental aneurysms (no previous history of subarachnoid haemorrhage (SAH)) of the anterior circulation (internal carotid, anterior cerebral, middle cerebral (including posterior communicating) arteries). (B) Incidental aneurysms of the posterior circulation and (C) additional aneurysms. [8] *J Neurol Neurosurg Psychiatry, 2005;76:234-9. Reproduced with permission from the BMJ Publishing Group.
Aneurysm screening
Genetic factors appear to be important in the causation of intracranial aneurysms. Therefore, screening relatives of SAH victims might seem an approach worthy of consideration. However, many factors need to be considered (Table 2).
In a useful review paper, White and Wardlaw conclude that only 156/21054 (0.74%) of first degree relatives sustain a SAH in population based longitudinal studies. [9] A recent Scottish study reported the 10-year prospective risk of sustaining a SAH for a first-degree relative of an index case as 1.2% (95% CI: 0.4 – 2%). The risk for second-degree relatives was 0.5% (CI: 0.1 – 0.8%). [10] There was a trend for the risk to be highest in families with two first-degree relatives affected and lowest if only one second degree relative was affected. On this basis screening for aneurysms should only be offered to patients over 30 years old in families with 2 or more first-degree relatives with a history of SAH. If only 1 first-degree relative has sustained a SAH, screening is not recommended. Other at risk patients (e.g. adult polycystic kidney disease) should be advised on an individual basis. Due to the high sensitivity and specificity, rapid acquisition time and low procedural risks (xray exposure) CT angiography is now our screening investigation of choice, replacing invasive digital subtraction angiography and MR angiography.
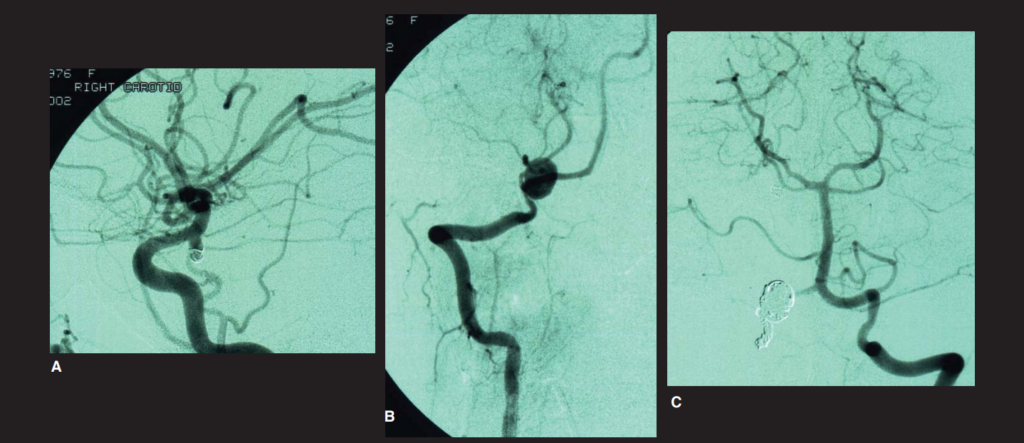
Summary and case study
High quality studies have helped to improve our knowledge of the natural history and treatment risks in patients with AUIAs. Knowledge of this literature is essential to formulate an evidence-based management plan applicable to the individual patient. Factors such as life expectancy, aneurysm location and size, coupled with the individual’s attitude to risk will govern the approach selected (Figure 3). Interdisciplinary teamwork between neurosurgeons and neuroradiologists is crucial to optimise evidence-based management. Widespread screening is not recommended and should be reserved for high-risk cases after discussion with the patient.
[Read more ACNR neurosurgery articles]
References
- Rinkel GJE, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture on intracranial aneurysms. A systematic review. Stroke 1998;29:251-6. https://doi.org/10.1161/01.STR.29.1.251
- Pobereskin LH. Incidence and outcome of subarachnoid haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry 2001;70:340-3. https://doi.org/10.1136/jnnp.70.3.340
- Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg 2000;93:379-87. https://doi.org/10.3171/jns.2000.93.3.0379
- The International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms – risk of rupture and risks of surgical intervention. New England J Med 1998;339:1725-33. https://doi.org/10.1056/NEJM199812103392401
- International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103-10.
https://doi.org/10.1016/S0140-6736(03)13860-3 - Kassell NF, Torner JC, Haley ECJ, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg 1990;73:18-36. https://doi.org/10.3171/jns.1990.73.1.0018
- Molyneux AJ, Kerr RSC, Yu L-M, Clarke M, Yarnold JA, Sandercock P. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups and aneurysm occlusion. Lancet 2005;366:809-17. https://doi.org/10.1016/S0140-6736(05)67214-5
- Vindlacheruvu RR, Mendelow AD, Mitchell P. Risk-benefit analysis of the treatment of unruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry 2005;76:234-9. https://doi.org/10.1136/jnnp.2003.031930
- White PM, Wardlaw JM. Unruptured intracranial aneurysms. Detection and management. J Neuroradiol 2003;30:336-50.
- Teasdale GM, Wardlaw JM, White PM, Murray G, Teasdale EM, Easton V. The familial risk of subarachnoid haemorrhage. Brain 2005;128:1677-85. https://doi.org/10.1093/brain/awh497
