Abstract
Since the late 80s with the discovery of oligo-clonal bands (OCBs) in the CSF of Multiple Sclerosis (MS) patients, scientists have made huge efforts to develop prognostic biomarkers in both the CSF and blood. In general terms, the latter has resulted in the development of either immune system activation/regulation biomarkers, or neurodegenerative biomarkers. Simply put, from a biomarker perspective, disease progres-sion in MS is due not only to the underlying autoimmunity, but neurodegeneration. As there has been resurgence of interest in the OCBs with the 2018 McDonald criteria, we discuss this first in the review.
Introduction
Multiple sclerosis (MS) is a progressive inflam-matory demyelinating disease of the central nervous system (CNS). It is now well accepted that Th1 and Th17 cells play an important role in the pathogenesis of MS, but contrary to belief, they are not the only cells involved. A combination of antibody-producing B cells/plasma cells, macrophages, and NK cells are involved in disease pathogenesis, whilst demyelination, inflammation and axonal damage contribute to progressive disability in MS patients. Over the last few years, scientists and clinicians have worked together in order to identify specific biomarkers able to predict the onset and course of the disease. However, despite the dramatic increase in publications, the biomarkers commonly used in clinical practice still remain the cerebrospinal fluid (CSF) oligoclonal bands, and more recently the neurofilament proteins. In this review we summarise the biomarkers that have made waves in MS research over the last ten years, including osteopontin, microRNAs, neurofilaments, chitinase and chitinase-like proteins. We also discuss oligoclonal bands, particularly as these have been reintroduced into the latest diagnostic criteria for MS.
Oligoclonal bands
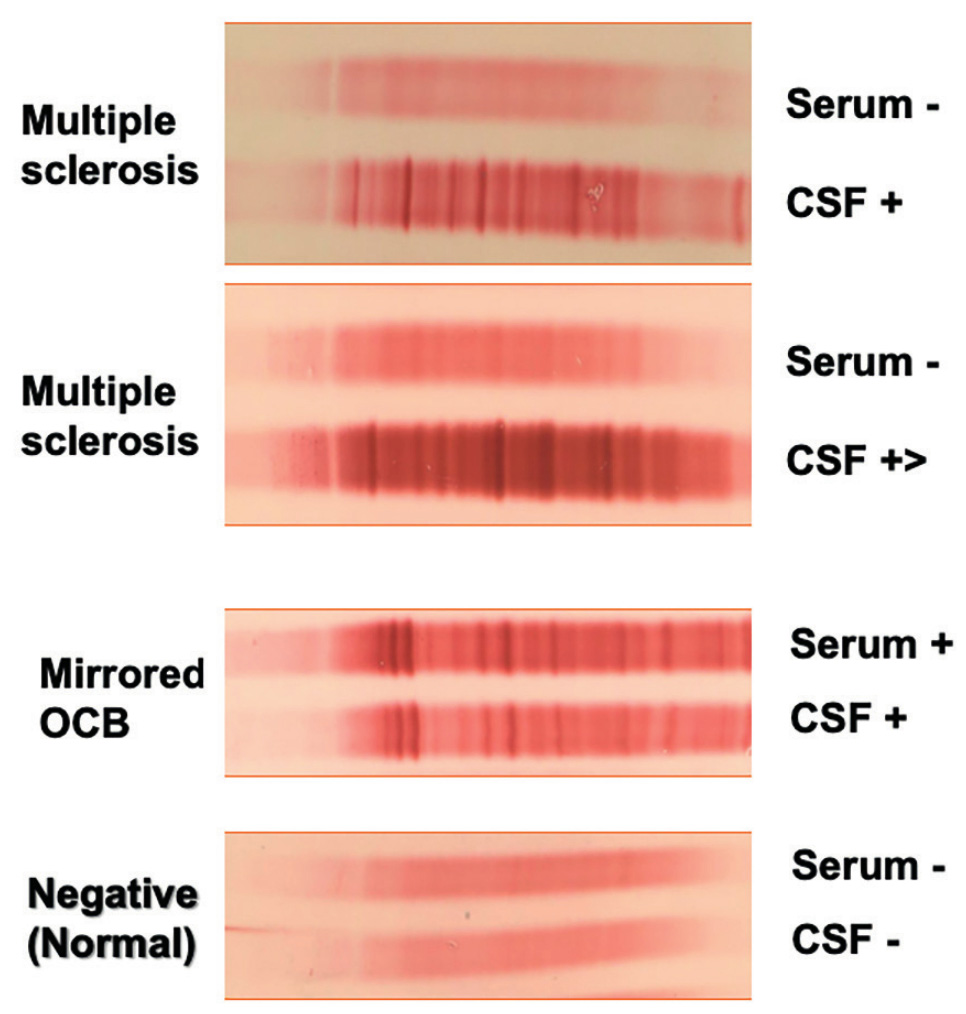
systemic source.
Immunoglobulin IgG oligoclonal bands (OCB) are detected in about 95% of MS patients and are considered the best diagnostic element supportive of MS diagnosis. Although OCBs are found mainly in CSF of people with MS, they also occur in other inflammatory conditions like paraneoplastic disorders, CNS lupus, neurosarcoidosis, Behcet’s disease and various forms of cerebral angiitis. OCB negative MS patients have been reported to have fewer infratentorial and more juxtacortical lesions compared to OCB-positive patients.1 OCB negativity is also associated with better prognoses based on physical disability.2
If we focus on the prognostic significance, Tintore et al. found that those who are OCB positive had a higher risk of conversion from a clinically isolated syndrome (CIS) to clinical definite MS.3 It therefore adds information to MRI in the first attacks of MS, and henceforth has been re-introduced back into the 2017 McDonald criteria.4 Several studies have also investigated the correlation between OCBs and cerebral volume. Ferreira et al., studied both grey and white matter volumes, and noted less brain atrophy in those who were OCB negative.5 While Fenu et al. found that brain atrophy in OCB positive patients primarily involved the white rather than grey matter.6
Osteopontin
Osteopontin (OPN) is a pleiotropic cytokine expressed by immune cells, including T cells, dendritic cells, macrophages and natural killer cells.7 It is involved in a variety of physiological functions and pathological states such as bone remodelling, wound healing, cancer biology and vascular disorders, and exerts pro-inflammatory and pro-angiogenic effects.8 OPN is considered to be a pro-inflammatory mediator that amplifies the inflammatory process by enhancing the production of interferon gamma (IFN-γ) and IL-17 from T cells with consequent inhibition of IL-10.9
Elevated OPN gene expression was found in MS brain lesions compared to control brain tissue10 and these findings were also confirmed in analysis of spinal cord tissue in experimental autoimmune encephalomyelitis (EAE).11 Many studies have reported increased concentration of CSF OPN in relapsing remitting MS (RRMS) patients compared to CIS and secondary progressive MS (SPMS) patients. Similarly, plasma OPN levels have been found to be increased in RRMS compared to healthy controls.12 However, a raised CSF OPN or blood OPN is not specific for MS, and has also been demonstrated in Alzheimer’s disease and Parkinson’s disease.13,14
MicroRNAs
Micro RNAs (miRNA) are short non-coding RNAs with an important role in post-transcriptional gene expression by silencing; via binding of the target messenger (mRNAs) or by degrading the mRNA transcript. MiRNAs play a major role in regulating key processes in immune cells, including Th1, Th17, T-regs,15 as well as being found in a number of neurological disorders, including traumatic CNS injuries.16 More recent findings suggest a role for miRNAs as biomarkers in MS.17 Huang et al. identified a link between dysregulated miRNAs and MS.18 Specifically, higher concentrations of miRNAs were observed in the serum of MS patients compared to controls19 and among these let-7i miRNA has been found to reduce the number of T-reg IFNy-IL17A-Fox3P-CD4+ cells, by targeting insulin like growth factor 1 receptor (IGF1R) and transforming growth factor beta receptor 1 (TGFBR1).20 The impairment of T Reg cells, with consequent disruption of the immune homeostasis, is considered crucial in the initiation and perpetuation of autoimmune disease.21
Neurofilaments
Neurofilaments, an abundant protein in the cytoskeleton neurons are composed of the subunits light (NfL; 60–70 kDa), medium (NfM; 130–170 kDa) and heavy chain (NfH; 180–200 kDa). Although the precise mechanism of axonal loss in MS is still not clear, it has been repeatedly demonstrated that neurofilaments are released into the blood and CSF of MS patients after episodes of relapses and with slow neurodegeneration. They are detectable in most at diagnosis, and even at the early stages of CIS and radiologically isolated syndrome (RIS).
The research has come a long way since MRI was the only tool available able to monitor the course of the MS status and there is the need to explore biomarkers that can accurately be detected at a very early stage of the disease. Among these biomarkers, NfL emerged as a favourable candidate. There are many reports backing its usefulness early on in prognostication, with increased levels predicting the development of MS in CIS and RIS.22
In MS, NfL levels in CSF and serum increase with EDSS, whilst the incremental rise correlates with lesion load and worsening EDSS.23 Following natalizumab use, a treatment effect on NfL levels has been demonstrated in the CSF but not in the serum, indicating a relationship between anti-inflammatory therapy and axonal damage resolution.24,25 However, with the increased sensitivity of Simoa platform, there has been new exciting research investigating NfL levels in the serum, raising the possibility of a blood biomarker. To date good correlations have been demonstrated between serum NfL and CSF NfL,26 MRI activity and disability in CIS patients. At a cohort level serum NfL have definite utility in monitoring treatment effect and reduced levels have been documented with interferon beta27 and fingolimod,28 and may be a useful surrogate marker of treatment efficacy in clinical trials. At an individual level, it’s long-term predictive capacity is uncertain.
NfH, like NfL is a bulk biomarker of neuronal damage and has been found to be elevated in optic neuritis,29 in RRMS and SPMS and correlates with EDSS in cross sectional and longitudinal studies.30,31 NfH levels have been demonstrated to improve following lamotrigine treatment in SPMS and phenytoin in optic neuritis; two neuroprotection studies in MS.32,33 Antibodies to NFL have also been identified in MS, with elevated levels in CIS, primary progressive MS (PPMS) and RRMS and have been linked with clinical disability and progressive disease course. Their significance in MS is as yet not known.
Chitinase and Chitinase-like proteins
Chitinase (chitinase 1, CHIT1) and chitinase-like protein (chitinase 3-like protein 1, CHI3L1 and 2, CHI3L2) are chitin-binding proteins that belong to the glycohydrolase family 18 and may be indicators of inflammation.
Recent evidence indicates CHIT1 gene expression is greater in chronic active MS lesions, infiltrated by microglia and macrophages, compared to expression in the rim of active MS lesions.34 CHIT1 CSF levels were significantly higher in RRMS compared to controls. Novakova et al. showed a reduction in CSF CHIT1 in fingolimod-treated MS patients switched from first-line DMTs but they did not find a similar trend in natalizumab-treated patients.35 Hinsinger et al. showed that chitinase-like proteins, CHI3L1 and CHI3L2 are highly expressed in white matter plaques, specifically in astrocytes and microglial cells of MS patients.36 CHI3L1 has drawn attention in that it has been observed to be increased in CIS cases converting to MS compared to non-converters37 and the same trend was not confirmed in RIS patients.38 This observation, together with the evidence that CSF CHI3L1 increase accordingly to the progression of MS may represent an alternative diagnostic value to discriminate progressive patients at a very early stage. Since OCB testing fails to detect intrathecal IgG synthesis in about 5% of MS cases,39 determination of CSF CHI3L1 levels by ELISA may represent a future alternative to be utilised in MS clinical practice. In addition, reduced CSF CHI3L1 has been observed after 12 months of natalizumab treatment,40 reflecting the initial observations that this is a biomarker of inflammation. CHI3L1, is not specific for MS and has been found to be elevated in cancer and rheumatoid arthritis.41
CHI3L2, on the other hand, was originally thought to be a secretory product of chondrocytes, and as such has only recently been studied in MS. Unlike CHI3L1, CSF CHI3L2 decreased in PPMS compared to RRMS patients.36 A rise in CHI3L2 observed in RRMS and also correlated with other biomarkers of inflammation and tissue damage such as NfL, OPN and MBP,42 suggesting like the others an association with inflammatory activity.
Conclusion
In the past, MS was considered to be an exclusively T-cell mediated-disease, but increasingly it is clear that we are dealing with a multifactorial disease pathogenesis leading to progressive disability. Understanding the role of each of these factors may allow for better definition of the under-lying predominant disease process in each patient, permitting more individualised therapeutic strategies. The drive to find new validated biomarkers in MS to facilitate this process has often had unpredictable results. We now understand that the majority of these biomarkers are either indicators of bulk tissue injury i.e. neurodegeneration or inflammation, or impaired immune regulation in MS.
References
- Huttner HB, Schellinger PD, Struffert T, Richter G, Engelhorn T, Bassemir T, et al. MRI criteria in MS patients with negative and positive oligoclonal bands: equal fulfillment of Barkhof’s criteria but different lesion patterns. J Neurol. 2009;256(7):1121-5.
- Joseph FG, Hirst CL, Pickersgill TP, Ben-Shlomo Y, Robertson NP, Scolding NJ. CSF oligoclonal band status informs prognosis in multiple sclerosis: a case control study of 100 patients. J Neurol Neurosurg Psychiatry. 2009;80(3):292-6.
- Tintoré M1, Rovira A, Río J, Tur C, Pelayo R, Nos C, Téllez N, Perkal H, Comabella M, Sastre-Garriga J, Montalban X. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? 2008.25;70(13 Pt 2):1079-83.
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302.
- Ferreira D, Voevodskaya O, Imrell K, Stawiarz L, Spulber G, Wahlund LO, et al. Multiple sclerosis patients lacking oligoclonal bands in the cerebrospinal fluid have less global and regional brain atrophy. Journal of Neuroimmunology. 2014;274(1-2):149-54.
- Fenu G, Lorefice L, Sechi V, Loi L, Contu F, Cabras F, Coghe G, Frau J, Secci MA, Melis C, Schirru L, Costa G, Melas V, Arru M, Barracciu MA, Marrosu MG, Cocco E. Brain volume in early MS patients with and without IgG oligoclonal bands in CSF. Mult Scler Relat Disord. 19:55-58.
- Chen G, Zhang X, Li R, Fang L, Niu X, Zheng Y, et al. Role of osteopontin in synovial Th17 differentiation in rheumatoid arthritis. Arthritis Rheum. 2010;62(10):2900-8.
- Junaid A,Moon MC, Harding GE, Zahradka P. Osteopontin localizes to the nucleus of 293 cells and asso-ciates with p1-like kinase-1. Am J physiol Cell Physiol 2007;292(2):c919–c926.
- Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287(5454):860-4.
- Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, et al. The influence of the proinflamma-tory cytokine, osteopontin, on autoimmune demyelinating disease. 2001;294(5547):1731-5.
- Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8(1):74-83.
- Vogt MH, Lopatinskaya L, Smits M, Polman CH, Nagelkerken L. Elevated osteopontin levels in active relapsing-remitting multiple sclerosis. Ann Neurol. 2003;53(6):819-22.
- Carecchio M, Comi C. The role of osteopontin in neurode-generative diseases. J Alzheimers Dis. 2011;25(2):179-85.
- Sun Y, Yin XS, Guo H, Han RK, He RD, Chi LJ. Elevated osteopontin levels in mild cognitive impairment and Alzheimer’s disease. Mediators Inflamm. 2013;2013:615745.
- Jeker LT, Bluestone JA. MicroRNA regulation of T-cell differentiation and function. Immunol Rev. 2013;253(1):65-81.
- Liu NK, Xu XM. MicroRNA in central nervous system trauma and degenerative disorders. Physiol Genomics. 2011;43(10):571-80.
- Ma X, Zhou J, Zhong Y, Jiang L, Mu P, Li Y, et al. Expression, regulation and function of microRNAs in multiple sclerosis. Int J Med Sci. 2014;11(8):810-8.
- Huang Q, Xiao B, Ma X, Qu M, Li Y, Nagarkatti P, et al. MicroRNAs associated with the pathogenesis of multiple sclerosis. J Neuroimmunol. 2016;295-296:148-61.
- Vistbakka J, Elovaara I, Lehtimaki T, Hagman S. Circulating microRNAs as biomarkers in progressive multiple sclerosis. Mult Scler J. 2017;23(3):403-12.
- Kimura K, Hohjoh H, Fukuoka M, Sato W, Oki S, Tomi C, et al. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun. 2018;9.
- Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune Immunology. 2006;117(3):289-300.
- Gaetani L, Hoglund K, Parnetti L, Pujol-Calderon F, Becker B, Eusebi P, et al. A new enzyme-linked immuno-sorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther. 2018;10(1):8.
- Norgren N, Sundstrom P, Svenningsson A, Rosengren L, Stigbrand T, Gunnarsson M. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. 2004;63(9):1586-90.
- Petzold A, Mondria T, Kuhle J, Rocca MA, Cornelissen J, te Boekhorst P, et al. Evidence for acute neurotoxicity after chemotherapy. Ann Neurol. 2010;68(6):806-15.
- Gunnarsson M, Malmestrom C, Axelsson M, Sundstrom P, Dahle C, Vrethem M, et al. Axonal Damage in Relapsing Multiple Sclerosis is Markedly Reduced by Natalizumab. Annals of Neurology. 2011;69(1):83-9.
- Piehl F, et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler 2018;24(8):1046-54.
- Kuhle J, Nourbakhsh B, Grant D, Morant S, Barro C, Yaldizli Ö, Pelletier D, Giovannoni G, Waubant E, Gnanapavan S. (2017). Serum neurofilament is associated with progression of brain atrophy and disability in early MS.
- Kuhle J, et al. Fingolimod and CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology 2015;84(16):1639-43. Neurology. 2017 Feb 28.
- Lim ET, Grant D, Pashenkov M, Keir G, Thompson EJ, Soderstrom M, et al. Cerebrospinal fluid levels of brain specific proteins in optic neuritis. Mult Scler. 2004;10(3):261-5.
- Teunissen CE, Iacobaeus E, Khademi M, Brundin L, Norgren N, Koel-Simmelink MJ, et al. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. 2009;72(15):1322-9.
- Kuhle J, Leppert D, Petzold A, Regeniter A, Schindler C, Mehling M, et al. Neurofilament heavy chain in CSF correlates with relapses and disability in multiple sclerosis. Neurology. 2011;76(14):1206-13.
- Gnanapavan S, Grant D, Morant S, Furby J, Hayton T, Teunissen CE, et al. Biomarker Report from the Phase II Lamotrigine Trial in Secondary Progressive MS – Neurofilament as a Surrogate of Disease Progression. Plos One. 2013;8(8).
- Gnanapavan S, Grant D, Raftopoulos R, Hickman S, Altmann D, Barro C, et al. Neurofilament results for the phase II neuroprotection study of phenytoin in optic neuritis. Mult Scler J. 2016;22:855-6.
- Hendrickx DAE, van Scheppingen J, van der Poel M, Bossers K, Schuurman KG, van Eden CG, et al. Gene Expression Profiling of Multiple Sclerosis Pathology Identifies Early Patterns of Demyelination Surrounding Chronic Active Lesions. Front Immunol. 2017;8:1810.
- Novakova L, Axelsson M, Khademi M, Zetterberg H, Blennow K, Malmestrom C, et al. Cerebrospinal fluid biomarkers as a measure of disease activity and treat-ment efficacy in relapsing-remitting multiple sclerosis. J 2017;141(2):296-304.
- Hinsinger G, Galeotti N, Nabholz N, Urbach S, Rigau V, Demattei C, et al. Chitinase 3-like proteins as diagnostic and prognostic biomarkers of multiple sclerosis. Mult Scler 2015;21(10):1251-61.
- Comabella M, Fernandez M, Martin R, Rivera-Vallve S, Borras E, Chiva C, et al. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. 2010;133(Pt 4):1082-93.
- Matute-Blanch C, et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain 2018;141(4):1085-93.
- Freedman MS, et al. (2005). Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol 2005;62(6): 865-70.
- Stoop MP, Singh V, Stingl C, Martin R, Khademi M, Olsson T, et al. Effects of Natalizumab Treatment on the Cerebrospinal Fluid Proteome of Multiple Sclerosis Patients. J Proteome Res. 2013;12(3):1101-7
- Tsuruha JI, Masuko-Hongo K, Kato T, Sakata M, Nakamura H, Sekine T, et al. Autoimmunity against YKL-39, a human cartilage derived protein, in patients with osteoarthritis. J Rheumatol. 2002;29(7):1459-66.
- Mollgaard M, Degn M, Sellebjerg F, Frederiksen JL, Modvig S. Cerebrospinal fluid chitinase-3-like 2 and chitotriosidase are potential prognostic biomarkers in early multiple sclerosis. Eur J Neurol. 2016;23(5):898-905.

