Abstract
Cerebral amyloid angiopathy (CAA) is increasingly recognised, particularly as a cause of intracerebral haemorrhage and dementia. CAA may present to the clinical neurologist in a range of circumstances, including inpatient or outpatient general neurology (with the subacute encephalopathy of CAA-related inflammation, or transient focal neurological episodes), dementia clinics (in particular in association with Alzheimer’s disease) and, of course, in the context of acute stroke (intracerebral haemorrhage). This clinical review article presents an overview of the key clinical, neuropathological and imaging findings in CAA, as well as a practical review of the challenging management aspects relevant to CAA-related intracerebral haemorrhage.
Introduction
Our concept of cerebral amyloid angiopathy (CAA) has radically evolved over time: considered a rare pathological curiosity in the early 20th century, CAA is now an increasingly recognised cause of cerebral haemorrhage and dementia, with important diagnostic and mechanistic implications.1 This development in our understanding was greatly facilitated by an improved ability to diagnose CAA in vivo, thanks to significant advances in neuroimaging.2-4 CAA usually presents to clinicians in one of four ways: lobar intracerebral haemorrhage (ICH); dementia or cognitive decline; transient focal neurological episodes; and the encephalopathy seen in acute CAA-related inflammation (Table 1).1,5
The neuropathological coexistence of CAA and Alzheimer’s disease (AD) is well recognised5 with pathological evidence of CAA in 80 – 98% of AD brains, but these processes can also occur independently of one another: only 50% of those with CAA meet the pathological criteria for AD, and moderate-to-severe CAA is seen in only 25% of those with AD.6,7 There is also a growing appreciation that the amyloid related imaging abnormalities (ARIA) seen in those with AD receiving amyloid beta (Aβ) immunotherapy bears a striking resemblance to inflammatory CAA, and that the extent of the response may be related to pre-treatment CAA severity, suggesting a role beyond that of innocent bystander in AD pathophysiology.8-11 The fact that non-Aβ amyloid proteins can also form comparable vascular deposits with similar clinical manifestations1 has led to a hypothesis that these conditions are all due to failures of normal perivascular protein elimination pathways,12 which may have therapeutic relevance in the future.
The first half of this short summary aims to introduce CAA by describing its characteristic neuropathological and imaging findings. The second half will explore the role of CAA in ICH, in particular our current diagnostic criteria and the potential management implications CAA has in the context of ICH.
What is cerebral amyloid angiopathy?
Neuropathology
CAA is one of the cerebral small vessel diseases, a broad term that describes any vascular pathology affecting the small (usually <2mm) arterioles, capillaries and venules of the brain.5,13 CAA particularly affects the cortical and leptomeningeal vessels of the cerebrum and cerebellum, frequently sparing deeper structures such as the basal ganglia, thalamus and brainstem.1,5 This progressive vascular deposition of amyloid protein has been described for eight types of amyloid protein, most of which have been identified because they cause inherited forms of CAA that tend to present with dementia or ICH.1,14 As the CAA secondary to Aβ is by far the most common,1,11 the remainder of this article will focus upon this subtype; subsequent references to CAA are to Aβ CAA.
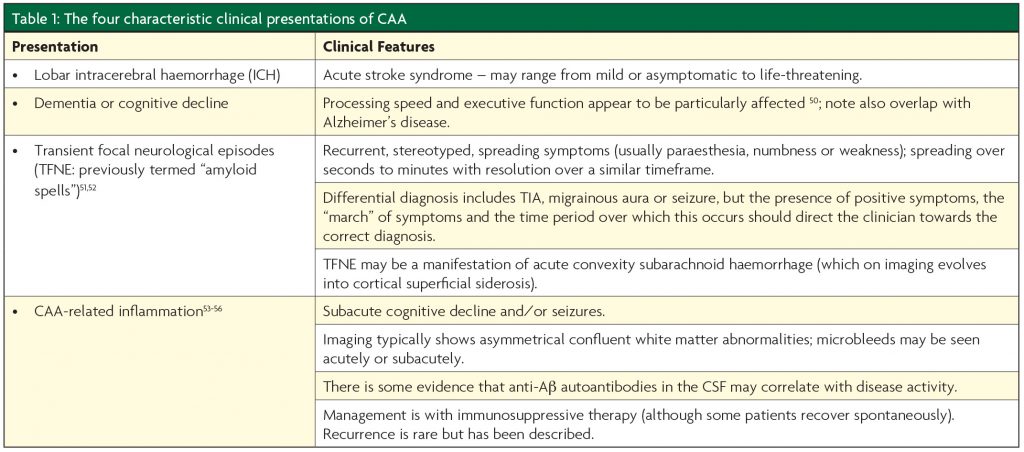
Aβ protein is formed from the Amyloid Precursor Protein (APP), with the 42 amino acid fragment found mainly in the parenchymal amyloid deposits characteristic of Alzheimer’s disease, and the 40 amino acid form tending to be deposited in the vasculature.5 Progressive accumulation of perivascular Aâ results in smooth muscle loss and eventual “double barrelling” (Figure 1).5
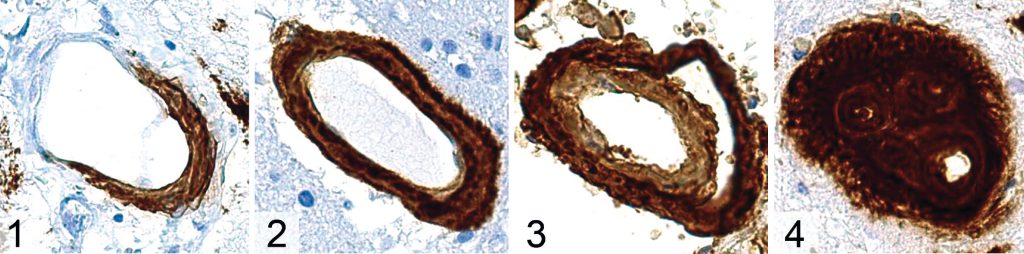
CAA can be subdivided based upon which type of vessel is affected, with type 1 CAA affecting capillaries as well as arterioles and venules, and type 2 being “capillary-sparing”, whilst affecting all other vessel types.1,5 Interestingly, these subtypes appear to be associated with specific alleles of Apolipoprotein E (ApoE) and may have discrete clinical manifestations.5 ApoEε2 seems to be associated with type 2 CAA,5 and is also seen more frequently in those with CAA and ICH, as well as those with disseminated cortical superficial siderosis15 (a haemorrhagic imaging marker of CAA2). ApoEε4, on the other hand, has been described as “the most prevalent genetic risk factor for sporadic AD”16 and is also associated with the cognitive decline observed in normal ageing.17 It seems to be associated with type 1 CAA pathologically, and CAA without ICH clinically.5,15 Mechanistically, this raises the possibility that the size of the affected vessel dictates clinical presentation, with capillary level disease tending to result in cognitive impairment and arteriolar level involvement resulting in ICH; further work is necessary in order to establish whether or not this is the case.
Imaging markers
The recent advances in our understanding of CAA have been made possible by the identification of new neuroimaging measures that allow a diagnosis to be made without pathological material.2 Although a number of novel imaging techniques, including diffusion tensor imaging, visual functional MRI and amyloid-PET, have diagnostic potential in CAA,2 these are not always widely available in clinical practice. Table 2 describes imaging markers of CAA that may be easily identified on standard clinical MR sequences, examples of which are shown in Figure 2.
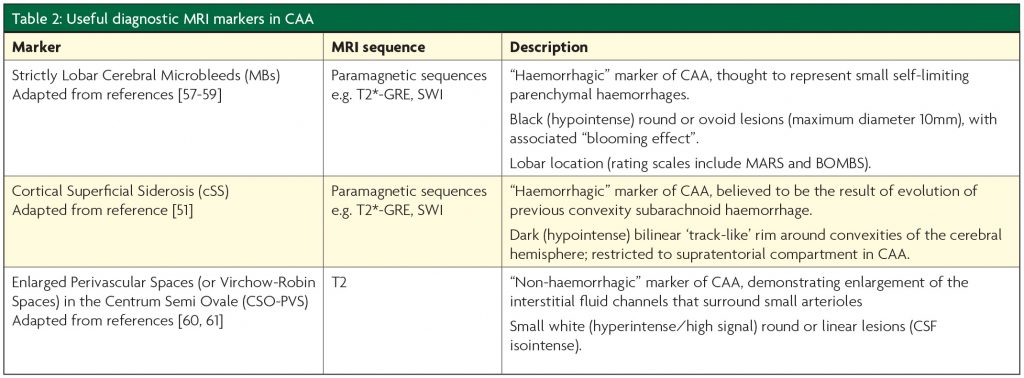
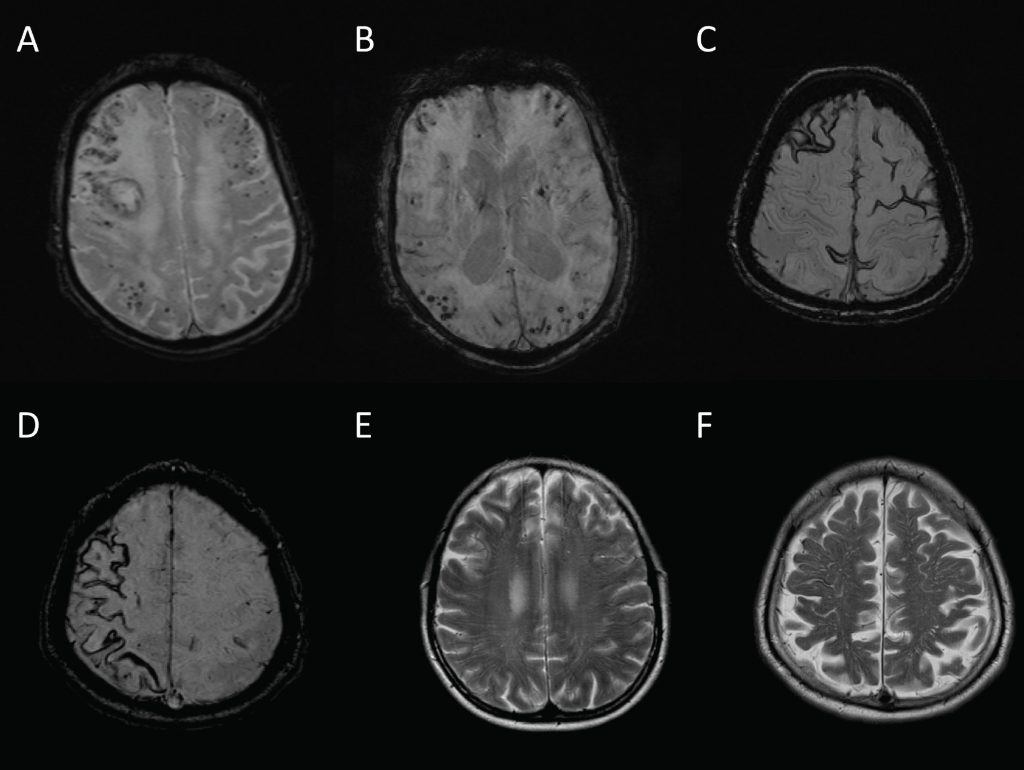
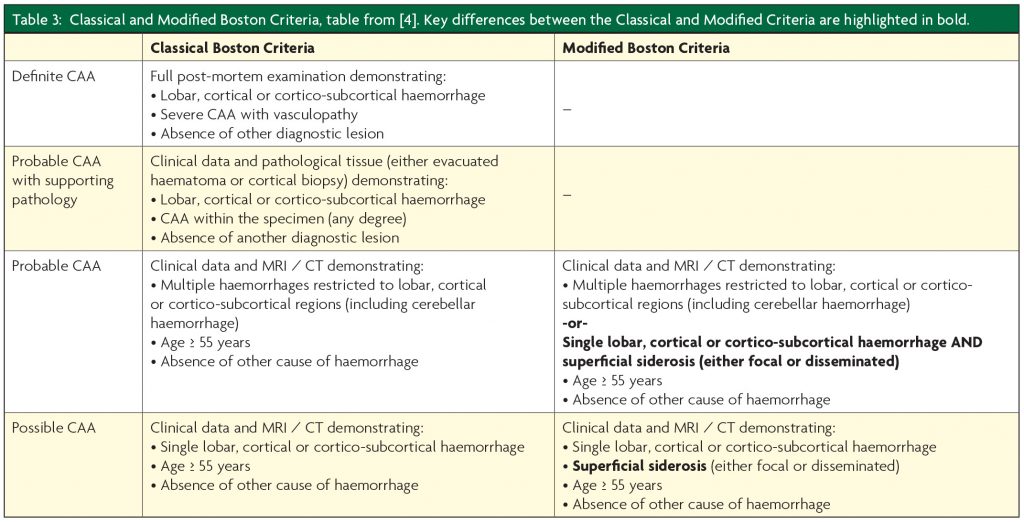
CAA is diagnosed using either the Classical or Modified Boston Criteria (Table 3).3,4 Given the increasing evidence for a “non-haemorrhagic” CAA phenotype, these criteria may require amendments so that those who may be “cognitive-predominant” (i.e. without macro- or microhaemorrhage) can still be accurately diagnosed.
CAA and ICH – what do we know, and what can be done?
The association between CAA and ICH, in particular lobar ICH, has been recognised for some time; a recent meta-analysis found a significant association between CAA and lobar ICH (OR 2.21, 95% CI 1.09 to 4.45).18 The fact that CAA is associated with lobar ICH in particular has significance, as lobar ICH appear to be more likely to recur, with an annual recurrence rate of between 2.5 – 14.3% compared with 1.3 – 2.9% for non-lobar ICH.19 Given that the estimated one year survival rate in those with ICH is 46%,19 and CAA may be responsible for up to 50% of lobar ICH,20 modifying this risk could have a dramatic effect on ICH rates.
The risk factors for CAA-related ICH can be considered as modifiable or non-modifiable. Non-modifiable risk factors include increasing age, Alzheimer’s disease, and any predisposing genetic factors (for example, inherited forms of CAA, or particular ApoE variants).1,21 The presence of CAA itself, perhaps the most obvious risk factor for CAA-related haemorrhage, has always been thought of as non-modifiable; the hope is that, with the development of new therapeutic strategies for CAA such as the anti Aβ-40 monoclonal antibody ponezumab,22 this will change.
The modifiable risk factors for CAA-related ICH are hypertension and the use of drugs that increase overall bleeding risk, for example antiplatelet agents, anticoagulants and thrombolytic strategies.1 Statin use may also be a modifiable risk factor in this situation. These factors will now be considered in turn.
The main evidence for blood pressure (BP) lowering in CAA comes from a sub-analysis of the PROGRESS trial.23 This study demonstrated that, even though those with CAA-related ICH tended to have lower BP than those with hypertension-related ICH (137/81mmHg vs 157/88mmHg respectively), it was the CAA group that seemed to benefit the most from BP reduction, with a 77% reduction in CAA-related ICH.23 Although PROGRESS did not have a target BP, the trials demonstrated reductions in stroke risk for both hypertensive (>160/90mmHg at baseline) and non-hypertensive groups; the latter group had a mean entry BP of 136/79mmHg and the average BP reduction in the treatment group was 9/4mmHg.24 Based on this, it seems reasonable to aim for a BP target of ~125/75mmHg, which is also in keeping with the results from SPS3, which showed a significant reduction in ICH in those with a BP less than 130/80mmHg.25 However, further randomised data in ICH survivors with an aggressive BP treatment target are needed to confirm safety and efficacy in this ICH population. A trial of telemetry-guided intensive BP control is in set up in the UK to address this (Prevention Of Hypertensive Injury to the Brain by Intensive Treatment–ICH – PROHIBIT-ICH, D Werring, personal communication).
As those with CAA are at increased risk of ICH, medications that impair normal haemostasis (antiplatelet drugs, anticoagulants, intravenous thrombolysis) are best avoided, although this is not always possible and presents a difficult clinical dilemma,26 especially as patients with CAA also appear to be at increased risk of ischaemic events.27 There is observational evidence in favour of avoiding anticoagulation with warfarin in CAA,28,29 and there are case reports of ICH in CAA following treatment with intravenous thrombolysis.30-32 Presence of the ApoEε2 allele seems to particularly be associated with warfarin related ICH.29,33,34 However, there are no randomised trial data to inform the use of warfarin in CAA. The role of non-vitamin K oral anticoagulants (with about half of the ICH risk of warfarin) in those with CAA and an indication for anticoagulation (e.g. atrial fibrillation) remains to be defined, but our practice at present is to avoid long term oral anticoagulants in CAA unless there is a clear unavoidable need to give them (e.g. metallic heart valves, life-threatening venous thromboembolism). For patients with atrial fibrillation, left atrial appendage occlusion (LAAO) may have a role in patients with CAA as it has similar efficacy to oral anticoagulation with warfarin, but without the need for long anticoagulation exposure.35-37 The case for antiplatelet agents as a clear risk factor for future ICH in CAA is less clear cut – aspirin has been the most widely studied, and has been suggested as both increasing the risk of ICH in CAA38 and as having no effect.28 In patients with vaso-occlusive disease and a clear ongoing indication for antiplatelet use (e.g. severe ischaemic heart disease) inclusion in the randomised trial RESTART (http://www.restarttrial. org) should be considered; however, clinicians may not have equipoise about possible benefit if patients have imaging evidence of severe CAA and a history of recurrent ICH. Further studies are required, but meanwhile care must be taken in how CAA is diagnosed; in particular, with regard to the use of cerebral microbleeds in diagnostic criteria, as these may be a consequence of antiplatelet or anticoagulant treatments in those with and without CAA.39,40
Whether statins increase the risk of future ICH in those with CAA remains uncertain. There is, however, some evidence that they are associated with an increase in microbleed frequency41,42. The evidence is conflicting:43-46 although there is observational evidence of an association between intracranial haemorrhage (macro- and micro-) with reduced LDL-cholesterol, convincing randomised evidence that lipid lowering can increase ICH risk remains scarce.43,47 A decision analysis suggested that in CAA-related ICH the risks of statins for future ICH might outweigh the benefit for prevention of vaso-occlusive disease, but that statins may be less hazardous in deep, non-CAA related ICH.48,49 It seems reasonable to avoid statins after CAA-related ICH unless there is a clear and compelling indication for benefit on overall vascular risk. Randomised trials are ideally needed, with ICH subtyping as CAA- or non-CAA, to resolve this controversial therapeutic dilemma.
Summary
- CAA is a small vessel disease that occurs as a consequence of vascular amyloid deposition
- CAA has a spectrum of disease, with haemorrhage-predominant and cognitive-predominant subtypes, which may be related to ApoE genotype
- New MRI markers greatly facilitate the diagnosis of CAA from standard MRI sequences
- Key management strategies are avoidance of anticoagulant medications and aggressive blood pressure control; the hazard of antiplatelet drugs and statins in those with CAA remains unclear
References
- Yamada M, Cerebral amyloid angiopathy: emerging concepts. Journal of stroke, 2015;17:17-30.
- Greenberg SM, Al-Shahi Salman R, Biessels GJ, van Buchem M, Cordonnier C, Lee JM, Montaner J, Schneider JA, Smith EE, Vernooij M, Werring DJ. Outcome markers for clinical trials in cerebral amyloid angiopathy, Lancet neurology, 2014;13:19-428.
- Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria, Neurology, 2001;56:537-9.
- Linn J1, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, van Buchem MA, Bruckmann H, Greenberg SM. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy, Neurology, 2010;74:1346-50.
- Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum, Journal of neurology, neurosurgery, and psychiatry, 2012;83:124-37.
- Jellinger KA, Alzheimer disease and cerebrovascular pathology: an update. Neural Transm., 2002;109:813-36.
- Viswanathan A, Greenberg SM. Cerebral Amyloid Angiopathy in the Elderly, Annals of neurology, 2011;70:871-80.
- Werring DJ, Sperling R, Inflammatory cerebral amyloid angiopathy and amyloid-modifying therapies: variations on the same ARIA?, Annals of neurology, 2013;73:439-41.
- Ryan NS, Lashley T, Revesz T, Dantu K, Fox NC, Morris HR. Spontaneous ARIA (amyloid-related imaging abnormalities) and cerebral amyloid angiopathy related inflammation in presenilin 1-associated familial Alzheimer’s disease, J. Alzheimers Dis., 2015;44:1069-74.
- Arrighi HM, Barakos J, Barkhof F, Tampieri D, Jack Jr C, Melancon D, Morris K, Ketter N, Liu E, Brashear HR. Amyloid-related imaging abnormalities-haemosiderin (ARIA-H) in patients with Alzheimer’s disease treated with bapineuzumab: a historical, prospective secondary analysis, Journal of neurology, neurosurgery, and psychiatry, 2015.
- Esiri M, Chance S, Joachim C, Warden D, Smallwood A, Sloan C, Christie S, Wilcock G, Smith AD. Cerebral amyloid angiopathy, subcortical white matter disease and dementia: literature review and study in OPTIMA, Brain Pathol., 2015;25:51-62.
- Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO. Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy, Neuropathology and applied neurobiology, 2013;39:593-611.
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges, The Lancet. Neurology, 2010;9:689-701.
- Ghiso J, Fossati S, Rostagno A. Amyloidosis associated with cerebral amyloid angiopathy: cell signaling pathways elicited in cerebral endothelial cells, Journal of Alzheimer’s disease: JAD, 2014;42 Suppl 3:S167-76.
- Charidimou A, Martinez-Ramirez S, Shoamanesh A, Oliveira-Filh Jo, Frosch M, Vashkevich A, Ayres A, Rosand J, Gurol ME, Greenberg SM, Viswanathan A. Cerebral amyloid angiopathy with and without hemorrhage: evidence for different disease pheno- types, Neurology, 2015;84:1206-12.
- Michaelson DM. APOE epsilon4: the most prevalent yet understudied risk factor for Alzheimer’s disease, Alzheimer’s & dementia : the journal of the Alzheimer’s Association, 2014;10:861-8.
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy, Rev. Neurol., 2013;9:106-18.
- Samarasekera N, Fonville A, Lerpiniere C, Farrall AJ, Wardlaw JM, White PM, Smith C, Al-Shahi Salman R. Influence of intracerebral hemorrhage location on incidence, charac- teristics, and outcome: population-based study, Stroke; a journal of cerebral circulation, 2015;46:361-8.
- Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haem- orrhage: systematic review and meta-analysis, Journal of neurology, neurosurgery, and psychiatry, 2014;85:660-7.
- Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage, Current atherosclerosis reports, 2012;14:300-6.
- Yamada M, Brain hemorrhages in cerebral amyloid angiopathy, Seminars in thrombosis and hemostasis, 2013;39:955-62.
- A Phase 2, Randomized, Double Blind Placebo Controlled Trial To Evaluate The Safety, Tolerability, Pharmacokinetics And Efficacy Of Pf-04360365 (Ponezumab) In Adult Subjects With Probable Cerebral Amyloid Angiopathy.
- Arima H1, Tzourio C, Anderson C, Woodward M, Bousser MG, MacMahon S, Neal B, Chalmers J; PROGRESS Collaborative Group. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial, Stroke; a journal of cerebral circulation, 2010;41:394-6.
- Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 indi- viduals with previous stroke or transient ischaemic attack, Lancet, 2001;358:1033-41.
- SPSS Group, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM, Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial, Lancet, 2013;382:507-15.
- Hofmeijer J, Kappelle LJ, Klijn CJ, Antithrombotic treatment and intracerebral haemorrhage: between Scylla and Charybdis, Neurol., 2015;15L250-6.
- Gregoire SM, Charidimou A, Gadapa N, Dolan E, Antoun N, Peeters A, Vandermeeren Y, Laloux P, Baron JC, Jager HR, Werring DJ. Acute ischaemic brain lesions in intracerebral haemorrhage: multicentre cross-sectional magnetic resonance imaging study, Brain : a journal of neurology, 2011;134:2376-86.
- van Etten ES, Auriel E, Haley KE, Ayres AM, Vashkevich A, Schwab KM, Rosand J, Viswanathan A, Greenberg SM, Gurol ME., Incidence of symptomatic hemorrhage in patients with lobar microbleeds, Stroke; a journal of cerebral circulation, 2014;45:2280-5.
- Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study, Neurology, 2000;55:947-51.
- Charidimou A, Nicoll JA, McCarron MO. Thrombolysis-related intracerebral hemor- rhage and cerebral amyloid angiopathy: accumulating evidence, Frontiers in neurology, 2015;6:99.
- McCarron MO, Nicoll JA. Cerebral amyloid angiopathy and thrombolysis-related intracere- bral haemorrhage, The Lancet. Neurology, 2004;3:484-92.
- Mattila OS, Sairanen T, Laakso E, Paetau A, Tanskanen M, Lindsberg PJ, Cerebral amyloid angiopathy related hemorrhage after stroke thrombolysis: case report and literature review, Neuropathology : official journal of the Japanese Society of Neuropathology, 2015;35:70-4.
- Falcone GJ, Radmanesh F, Brouwers HB, Battey TW, Devan WJ, Valant V, Raffeld MR, Chitsike LP, Ayres AM, Schwab K, Goldstein JN, Viswanathan A, Greenberg SM, Selim M, Meschia JF, Brown DL, Worrall BB, Silliman SL, Tirschwell DL, Flaherty ML, Martini SR, Deka R, Biffi A, Kraft P, Woo D, Rosand J, Anderson CD; International Stroke Genetics Consortium. APOE epsilon variants increase risk of warfarin-related intracerebral hemor- rhage, Neurology, 2014;83:1139-46.
- McCarron MO, Nicoll JA, Ironside JW, Love S, Alberts MJ, Bone I. Cerebral amyloid angi- opathy-related hemorrhage. Interaction of APOE epsilon2 with putative clinical risk factors, Stroke; a journal of cerebral circulation, 1999;30:1643-6.
- Horstmann S, Zugck C, Krumsdorf U, Rizos T, Rauch G, Geis N, Hardt S, Veltkamp R. Left atrial appendage occlusion in atrial fibrillation after intracranial hemorrhage, Neurology, 2014;82:135-8.
- Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P, Huber K, Whisenant B, Kar S, Swarup V, Gordon N, Holmes D. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial, Jama, 2014;312:1988-98.
- Wiebe J, Franke J, Lehn K, Hofmann I, Vaskelyte L, Bertog S, Sievert H. Percutaneous Left Atrial Appendage Closure With the Watchman Device: Long-Term Results Up to 5 Years, JACC Cardiovasc. Interv., 2015;8:1915-21.
- Biffi A, Halpin A, Towfighi A, Gilson A, Busl K, Rost N, Smith EE, Greenberg MS, Rosand J, Viswanathan A, Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angi- opathy, Neurology, 75 (2010) 693-698.
- Orken DN, Uysal E, Timer E, Kuloglu-Pazarci N, Mumcu S, Forta H, New cerebral microb- leeds in ischemic stroke patients on warfarin treatment: two-year follow-up, Clinical neur- ology and neurosurgery, 2013;115:1682-5.
- Vernooij MW, Haag MD, van der Lugt A, Hofman A, Krestin GP, Stricker BH, Breteler MM, Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study, Arch. Neurol., 2009;66:714-20.
- Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, Kase CS, Wolf PA, Seshadri S. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study, Stroke; a journal of cerebral circulation, 2014;45:1492-4.
- Haussen DC, Henninger N, Kumar S, Selim M, Statin use and microbleeds in patients with spontaneous intracerebral hemorrhage, Stroke; a journal of cerebral circulation, 2012;43:677-2681.
- Amarenco P, Bogousslavsky J, Callahan 3rd A, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA, High-dose atorvastatin after stroke or transient ischemic attack, The New England journal of medicine, 2006;355:549-59.
- Asberg S, Eriksson M. Statin therapy and the risk of intracerebral haemorrhage: a nationwide observational study, International journal of stroke : official journal of the International Stroke Society, 2015;10 Suppl A100:46-9.
- Jung JM, Choi JY, Kim HJ, Seo WK, Statin use in spontaneous intracerebral hemorrhage: a systematic review and meta-analysis, International journal of stroke : official journal of the International Stroke Society, 2015;10 Suppl A100:10-17.
- Tapia Perez JH, Yildiz OC, Schneider T, Nimsky C. Meta-analysis of Statin Use for the Acute Therapy of Spontaneous Intracerebral Hemorrhage, J. Stroke Cerebrovasc. Dis., 2015;24:2521-6.
- Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovas- cular disease or other high-risk conditions, Lancet, 2004;363:757-67.
- Lauer A, Greenberg SM, Gurol ME. Statins in Intracerebral Hemorrhage, Current athero- sclerosis reports, 2015;17:46.
- Westover MB, Bianchi MT, Eckman MH, Greenberg SM. Statin use following intracerebral hemorrhage: a decision analysis, Archives of neurology, 2011;68:573-9.
- Xiong L, Davidsdottir S, Reijmer YD, Shoamanesh A, Roongpiboonsopit D, Thanprasertsuk S, Martinez-Ramirez S, Charidimou A, Ayres AM, Fotiadis P, Gurol E, Blacker DL, Greenberg SM, Viswanathan A. Cognitive Profile and its Association with Neuroimaging Markers of Non-Demented Cerebral Amyloid Angiopathy Patients in a Stroke Unit, J. Alzheimers Dis., (2016).
- Charidimou A, Linn J, Vernooij MW, Opherk C, Akoudad S, Baron JC, Greenberg SM, Jäger HR, Werring DJ. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions, Brain : a journal of neurology, 2015;138:2126-39.
- L. Calviere, V. Cuvinciuc, N. Raposo, A. Faury, C. Cognard, V. Larrue, A. Viguier, F. Bonneville, Acute Convexity Subarachnoid Hemorrhage Related to Cerebral Amyloid Angiopathy: Clinicoradiological Features and Outcome, J. Stroke Cerebrovasc. Dis., 2016;25:1009-16.
- A. Charidimou, Cerebral Amyloid Angiopathy-Related Inflammation Biomarkers: Where are we Now?, J. Alzheimers Dis., 2016;50:9-11.
- J.A. Eng, M.P. Frosch, K. Choi, G.W. Rebeck, S.M. Greenberg, Clinical manifestations of cerebral amyloid angiopathy-related inflammation, Annals of neurology,2004;55:250-6.
- F. Piazza, S.M. Greenberg, M. Savoiardo, M. Gardinetti, L. Chiapparini, I. Raicher, R. Nitrini, H. Sakaguchi, M. Brioschi, G. Billo, A. Colombo, F. Lanzani, G. Piscosquito, M.R. Carriero, G. Giaccone, F. Tagliavini, C. Ferrarese, J.C. DiFrancesco, Anti-amyloid beta autoantibodies in cerebral amyloid angiopathy-related inflammation: implications for amyloid-modifying therapies, Annals of neurology, 2013;73:449-58.
- DiFrancesco JC, Touat M, Caulo M, Gallucci M, Garcin B, Levy R, Uncini A, Piazza F. Recurrence of Cerebral Amyloid Angiopathy-Related Inflammation: A Report of Two Cases from the iCAbeta International Network, Alzheimers Dis., 2015;46:1071-7.
- Yakushiji Y, Cerebral Microbleeds: Detection, Associations and Clinical Implications, Front. Neurosci., 2015;37:78-92.
- Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jager HR, Werring DJ, The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds, Neurology, 2009;73:1759-66.
- Cordonnier C, Potter GM, Jackson CA, Doubal F, Keir S, Sudlow CL, Wardlaw JM, Al-Shahi Salman R. improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS), Stroke; a journal of cerebral circulation, 2009;40:94-9.
- Charidimou A, Meegahage R, Fox Z, Peeters A, Vandermeeren Y, Laloux P, Baron JC, Jager HR, Werring DJ, Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study, Journal of neurology, neuro- surgery, and psychiatry, 2013;84:624-9.
- Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, Wardlaw JM, Enlarged perivascular spaces and cerebral small vessel disease, International journal of stroke: official journal of the International Stroke Society, 2015;10:376-81.

