Abstract
Chronic traumatic encephalopathy (CTE) is a neurodegenerative tauopathy associated with cumulative, prolonged exposure to symptomatic (concussive) or asymptomatic (subconcussive) repetitive head injuries (RHIs). An exposure-response effect has been demonstrated in American football along with case reports in high profile sports including Australian rules football, rugby union, rugby league, soccer, and ice hockey. Despite intense media interest in the professional contact and collision sports in which CTE has been demonstrated, CTE has been increasingly identified in a wide array of amateur sporting and also non-sporting environments including military related blast trauma, assault, and falls in this heterogeneous condition. Formerly thought a condition restricted to boxers in low case numbers, the clinical relevance of CTE neuropathological findings in footballers has become controversial in the new millennium, while nascent clinical and histopathological criteria are met by preliminary prospective biomarker studies. This article will explore aspects of clinicopathological controversies, the natural history of CTE, and frameworks for diagnosis and monitoring.
Shifting research scope
The field of CTE research has seen immense interest following the first report of CTE in American football by Omalu [1]. Prior to this, boxing was thought the main arena for developing CTE with an initial report by Martland in 1928 [2] on dementia pugilistica or punch-drunk syndrome before Critchley [3] coined the term CTE in 1957. Participants of contact collision sports [4–6], military service [7–9] or other trauma-related environments [10] are at risk of acquiring this neurodegenerative tauopathy caused by the cumulative neuropathological effect of repetitive head impacts (RHIs) [11–13]. A landmark study of 202 US football players found that 87% of the brains obtained were CTE positive [14]. The mean duration of play in mild and severe CTE pathology was 13 years (SD, 4.2 years) and 15.8 years (SD, 5.3 years) respectively. In 2013 a major push to understand the neuropathology of CTE was launched by the National Institutes of Health (NIH), supported by the Foundation for NIH’s Sports Health Research Program with funding from the National Football League (NFL). The first NINDS/National Institute of Biomedical imaging and Engineering (NIBIB) consensus panel met in February 2015 [15] followed by the second consensus panel 2021 [16] and following this the former categorisation of CTE into stages I-IV was superseded into two stages; ‘low-CTE’ where neuronal p-tau, in the form of neurofibrillary tangles, is restricted to one or more neocortical or subsulcal areas, and ‘high CTE’ where similar p-tau pathology is more widely distributed throughout the neocortex as well as other brain regions such the hippocampus, amygdala, thalamus, and cerebellar dentate nucleus, but the molecular mechanisms underlying this remain unclear.
Natural history of Chronic Traumatic Encephalopathy
Whilst single mild traumatic brain injury may be associated with prolonged impairment and disability, RHIs are associated with a cumulative worsening in cognitive, motor or behavioural outcomes [17,18]. The onset of an acquired neurodegenerative picture after RHIs across single or multiple domains is suggested by cognitive, psychological and behavioural decline over years in patients subsequently shown to have CTE [14,19,20]. Although the clinician may be confident that a patient presents with dementia, identifying the CTE subtype remains problematic in the absence of definitive biomarkers, and the correlation of in-life symptoms to the temporal onset of CTE neuropathology is still controversial [21–23]. Symptoms such as depression, anxiety and memory loss are often non-specific, subtle, may be treatment resistant, or are easily confounded by other factors. For these reasons, the term Traumatic Encephalopathy Syndrome (TES) is used to describe the in-life syndrome of CTE under revised NIH/National Institute of Neurological Disorders and Stroke (NINDS) research criteria [19]. For practical neurology purposes, the term CTE will also be used to describe the clinical manifestation in this article.
Progressive neuropsychiatric symptoms in CTE may associate with an increased suicide risk [24], although this remains controversial and unclear [25]. The Australian Sports Brain Bank (ASBB) has reported CTE in Australian rules football and rugby league with 12 of 21 former athletes who donated their brains for research being confirmed to have CTE. Of the 12 with CTE, 6 died by suicide [26]. Stern et al. (2013) described early data on survival, with mean symptom onset at 57.7 years (SD 5 18.3; range 25–82), mean dementia diagnosis 72.6 years (SD 5 8.5, range 56–83), and mean duration from diagnosis to death 8.0 years (SD 5 5.5, range 1–15) [27]. This would indicate a natural history of longer duration in CTE than many subtypes of dementia, with one large systematic review and meta analysis finding a mean survival from diagnosis in Alzheimer’s disease (AD) of only 5.8 years (SD 2.0) versus non-AD survival from diagnosis (MD −1·12 years, 95% CI −1·52 to −0·72) [28]. In such an insidious disorder of younger people where dementia may not be suspected, it is possible that CTE has until now “flown under the radar” of standard practice. Interpreting the natural history of a patient presenting with possible CTE may assist in timely diagnosis, remembering the characteristic younger onset explosive neuropsychiatric and delayed cognitive features on a background of prolonged impairment.
No study has confirmed the prevalence of CTE in the general population, but a dose exposure-effect is described [20,21]. Although all-cause mortality was lessened, theoretically due to a cardiovascular benefit with sport, higher rates of dementia mortality in soccer and dementia, Parkinson’s disease and motor neuron disease in rugby union have been shown from epidemiological studies [29–31]. Early work suggests that CTE in professional sports is just the “tip-of-the-iceberg” in these popular pursuits, with one study finding that 91% of college players and 21% of high school players had CTE [14], and another adjusting for selection bias suggesting that around 10% of professional American footballers may suffer CTE [32]. The risk of CTE may increase according to earlier age of exposure as well as total duration of exposure in the order of a trebling for every 3 years of play and earlier manifestation of cognitive and neuropsychiatric features with play before age 12 [20]. A causal relationship between RHIs and CTE using the Bradford-Hill criteria has been newly established [11].
Patients with CTE are likely to face decades of future potential increased health burden marked by psychiatric and neurological morbidity consistent with the literature, early mortality, and carer distress. In 36 individuals with CTE a bimodal age distribution of neuropsychiatric presentation was found in the younger group and cognitive presentation in the older group [27]. Symptoms such as emotional lability, poor impulse control, memory loss, headache, language deficits, visuospatial difficulties, executive dysfunction, and global cognitive decline are reported, and this neuropsychiatric flavour is supported in subsequent studies. Single mild traumatic brain injury is often undisclosed by individuals, yet when apparent it constitutes a high burden of acute care [33]. The potential health burden of CTE from other causes such as intimate partner violence is coming into sharper focus [10].
Taking the Trauma History
Due to increasing public awareness, use of the term CTE has become mainstream by patients and practitioners alike, and may be a more practical term for in-life symptoms than TES. A categorical approach to the history and examination with objective decline are needed to confirm features of CTE, which may evolve in those as young as 25 [34]. It is important to ask the age of onset of exposure and age of retirement, any missed seasons from sport, and any head injuries outside of sport. It can be helpful to provide a medical definition of concussion that includes common signs and symptoms beyond the commonly held belief that concussion is solely a ‘knock-out’ or loss of consciousness. Ataxia and bradykinesia, convulsion, confusion and poor responsivity can be helpful, immediate signs and the opportunity to educate patients and their families around these terms is often beneficial for future concussions.
Discussion may also centre around postconcussion syndrome, acute post-traumatic headache, delayed-onset persistent headache attributable to mild traumatic brain injury (International Classification of Headache Disorders ICD-3) and post-traumatic stress disorder due to assault or misadventure. Overlap with chronic migraine in patients with CTE can yield an opportunity for therapeutic intervention. Around one third of CTE patients will present with depression, and patients may either have symptoms at the time of retirement from contact or collision sport or in a delayed fashion [6]. Obstructive sleep apnoea, depression, attention deficit disorder, or other causes of cognitive impairment should be targeted for management, preferably before neuropsychological baseline.
The importance of repeated evaluation and a multidisciplinary approach similar to other dementia management, encompassing a neuropsychological baseline to inform individualised strategies for the management of these complex patients, cannot be overstated. Contextualising CTE within a spectrum of illness seen after RHIs and reinforcing that not everyone exposed to RHIs will develop CTE can alleviate anxiety, especially in those of lower risk. Obtaining the collateral history to detect cognitive and neuropsychiatric manifestations is needed as patients are often impaired for insight, and neurological monitoring 1-2 yearly in those with suspected CTE is recommended. CTE follows affective, temporal, frontal, and global constructs familiar to the clinician encountering other neurodegenerative illnesses in the memory clinic, but a prominence of explosivity to minimal psychosocial triggers is seen, in the absence of evidence for other disorders. In our practice, we actively enquire about road-rage, queue-rage, verbal or physical explosivity, irritability and intolerance. Consideration of the safety of children within the family unit of a patient with suspected CTE and explosivity should be a priority. Mood stabilisation with agents such as lamotrigine or sodium valproate and low-dose antipsychotic therapy can routinely be considered as with other disorders of emotional lability and cholinesterase inhibitors in those with suspected AD overlap.
Frontotemporal dementia, dementia with Lewy bodies and Parkinson’s plus disorders should be considered within the differential. Magnetic Resonance Imaging and fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) scanning of the brain can be used to delineate subtypes other than CTE, for which there is no definitive test in life. Lumbar puncture for the exclusion of autoimmune encephalitis, Creutzfeldt-Jakob Disease, AD, infective, inflammatory or neoplastic aetiologies should be considered in addition to electroencephalogram (EEG) or other secondary testing.
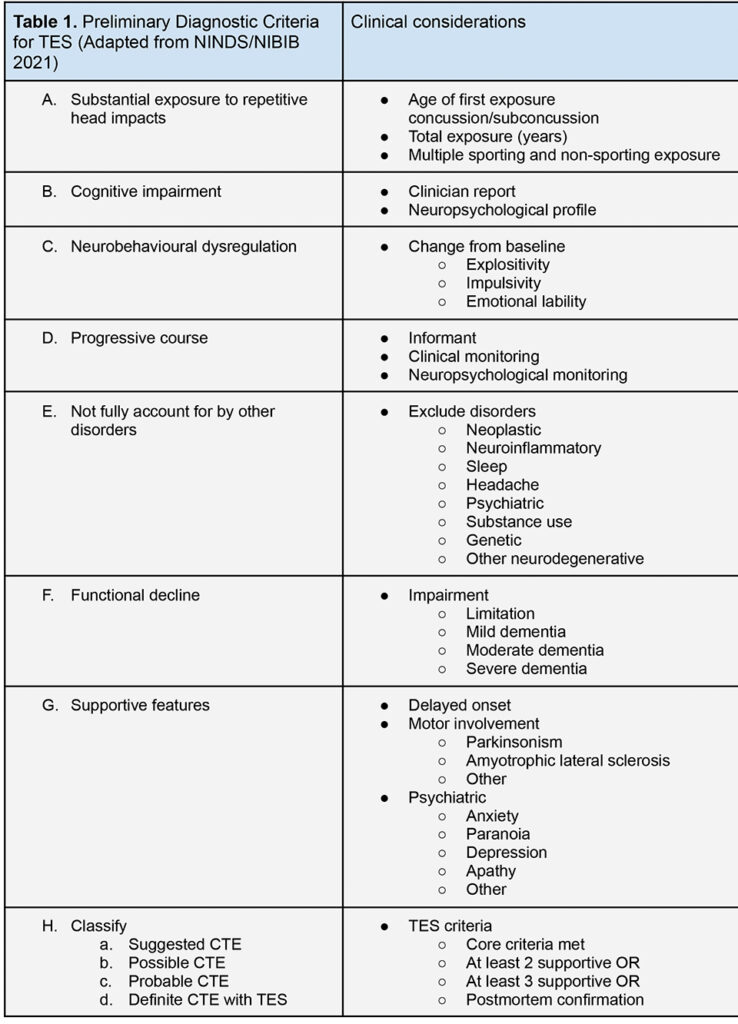
Proposed screening, diagnostic and monitoring guidelines
Although concussion is often highlighted as a priority area for sporting protocol adaptation, accumulated RHIs and subconcussion remain the target for reducing the risk of CTE, recognising that concussion history is confounded by recall bias, poor detection, motivation to stay and play, and misinterpretation of what concussion constitutes. Evidence based recommendations (EBRs) and high quality clinical guidelines for CTE are not yet developed, requiring multicentre collaborative research to determine useful biomarkers for studying CTE in life. However, consensus based recommendations and practice points enable a useful interim step to improving outcomes in CTE.
Given the weight of evidence for CTE as a disease entity and need for a precautionary approach there should be no delay in taking steps towards preventative action. First age of commencement of contact should be reviewed by all risky sports, including cycling, extreme sports, equestrian and others beyond football, balanced by the need to obtain safety skills for tackling or falling appropriately best learned during motor development. The cardiovascular and social benefits of sport can remain during this transitional phase, training risk can be reduced, and protocols modified such as those by the Football Association in the UK (no heading under 12) or the pioneering Tackle Can Wait programme in the US. These approaches are built upon the recognition that the developing brain is vulnerable, and that exposure prior to age 12 appears to predict an earlier cognitive (p < 0.0001) and behavioural/mood (p < 0.0001) symptom onset by 13.39 and 13.28 years, respectively [5].
A new dilemma arises regarding the screening of individuals already at risk of CTE. The global population facing dementia is expected to reach 130 million by 2050. It is currently not recommended to screen the general population for dementia [35], however there is precedent interest in the benefit of early detection and risk modification in subpopulations, such as those with vascular risk [36]. The NIH/NINDS identify five or more years of organised environmental exposure in American football, including two years at high school level or above, as primary diagnostic criteria for TES. In current neurological practice, the monitoring of existing players that befit criteria for CTE could reasonably be performed five yearly, plus consideration of repeated neuroradiological and neuropsychological evaluation. Performing a baseline neurological assessment as athletes enter the elite or heavy contact environments might be integrated into industry, insurance, medical or governance frameworks.
Conclusion
Chronic traumatic encephalopathy should be considered in any patient with more than five years of repetitive mild traumatic brain injury exposure and a self- or informant-reported decline in daily functioning, behavioural dysregulation, and cognitive performance, guided by an increasing index of suspicion for those with a higher accumulated burden of RHIs. Neurological evaluation, neuropsychological testing and monitoring can assist in identifying the at-risk patient as well as individualised strategies for care and monitoring. Traumatic encephalopathy syndrome is both an old and new entity, from Harrison Martland’s 1928 case reports to modern neuroscientific analysis. As with other neurodegenerative diseases, CTE can only definitively be confirmed neuropathologically, and finding sensitive and specific biomarkers presents an important and attainable goal for current research.
References
- Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128-34; discussion 128-34. https://doi.org/10.1227/01.NEU.0000163407.92769.ED
- Changa AR, Vietrogoski RA, Carmel PW. Dr Harrison Martland and the history of punch drunk syndrome. Brain. 2018;141:318-321. https://doi.org/10.1093/brain/awx349
- Critchley M. Punch-drunk syndromes: the chronic traumatic encephalopathy of boxers. Hommage a Clovis Vincent. 1949.
- Phelps A, Alosco ML, Baucom Z, Hartlage K, Palmisano JN, Weuve J, et al. Association of Playing College American Football With Long-term Health Outcomes and Mortality. JAMA Netw Open. 2022;5:e228775. https://doi.org/10.1001/jamanetworkopen.2022.8775
- Alosco ML, Mez J, Tripodis Y, Kiernan PT, Abdolmohammadi B, Murphy L, et al. Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol. 2018;83:886-901. https://doi.org/10.1002/ana.25245
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709-735. https://doi.org/10.1097/NEN.0b013e3181a9d503
- McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43-64. https://doi.org/10.1093/brain/aws307
- McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10: S242-53. https://doi.org/10.1016/j.jalz.2014.04.003
- Goldstein L. Chapter 5 – Mechanisms of Concussion, Traumatic Brain Injury, and Chronic Traumatic Encephalopathy: Acute and Chronic Effects of Blast Exposure. In: Budson AE, Mckee AC, Cantu RC, Stern RA, editors. Chronic Traumatic Encephalopathy. Elsevier. 2018;63-73. https://doi.org/10.1016/B978-0-323-54425-2.00005-9
- Costello K, Greenwald BD. Update on Domestic Violence and Traumatic Brain Injury: A Narrative Review. Brain Sci. 2022;12. https://doi.org/10.3390/brainsci12010122
- Nowinski CJ, Bureau SC, Buckland ME, Curtis MA, Daneshvar DH, Faull RLM, et al. Applying the Bradford Hill Criteria for Causation to Repetitive Head Impacts and Chronic Traumatic Encephalopathy. Front Neurol. 2022;13: 938163. https://doi.org/10.3389/fneur.2022.938163
- McKee AC. The Neuropathology of Chronic Traumatic Encephalopathy: The Status of the Literature. Semin Neurol. 2020;40:359-369. https://doi.org/10.1055/s-0040-1713632
- Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto-Ortolaza A, Johnston AE, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015;130:77-889. https://doi.org/10.1007/s00401-015-1502-4
- Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR, et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA. 2017;318:360-370. https://doi.org/10.1001/jama.2017.8334
- McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75-86. https://doi.org/10.1007/s00401-015-1515-z
- Bieniek KF, Cairns NJ, Crary JF, Dickson DW, Folkerth RD, Keene CD, et al. The Second NINDS/NIBIB Consensus Meeting to Define Neuropathological Criteria for the Diagnosis of Chronic Traumatic Encephalopathy. J Neuropathol Exp Neurol. 2021. https://doi.org/10.1093/jnen/nlab001
- Wright DK, Brady RD, Kamnaksh A, Trezise J, Sun M, McDonald SJ, et al. Repeated mild traumatic brain injuries induce persistent changes in plasma protein and magnetic resonance imaging biomarkers in the rat. Sci Rep. 2019;9: 1-13. doi:10.1038/s41598-019-51267-w https://doi.org/10.1038/s41598-019-51267-w
- Kulkarni P, Morrison TR, Cai X, Iriah S, Simon N, Sabrick J, et al. Neuroradiological Changes Following Single or Repetitive Mild TBI. Front Syst Neurosci. 2019;13: 34. https://doi.org/10.3389/fnsys.2019.00034
- Katz DI, Bernick C, Dodick DW, Mez J, Mariani ML, Adler CH, et al. National Institute of Neurological Disorders and Stroke Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome. Neurology. 2021. https://doi.org/10.1212/WNL.0000000000011850
- Mez J, Daneshvar DH, Abdolmohammadi B, Chua AS, Alosco ML, Kiernan PT, et al. Duration of American Football Play and Chronic Traumatic Encephalopathy. Ann Neurol. 2019. https://doi.org/10.1002/ana.25611
- Daneshvar, D.H., Nair, E.S., Baucom, Z.H. et al. Leveraging football accelerometer data to quantify associations between repetitive head impacts and chronic traumatic encephalopathy in males. Nat Commun 14, 3470 (2023). https://doi.org/10.1038/s41467-023-39183-0
- Iverson GL, Gardner AJ. Risk of Misdiagnosing Chronic Traumatic Encephalopathy in Men With Depression. J Neuropsychiatry Clin Neurosci. 2020;32:139-146. https://doi.org/10.1176/appi.neuropsych.19010021
- McCann H, Bahar AY, Burkhardt K, Gardner AJ, Halliday GM, Iverson GL, et al. Prevalence of chronic traumatic encephalopathy in the Sydney Brain Bank. Brain Commun. 2022;4:fcac189. https://doi.org/10.1093/braincomms/fcac189
- Iverson GL. Retired National Football League Players are Not at Greater Risk for Suicide. Arch Clin Neuropsychol. 2020;35:332-341. https://doi.org/10.1093/arclin/acz023
- Costanza A, Radomska M, Zenga F, Amerio A, Aguglia A, Serafini G, et al. Severe Suicidality in Athletes with Chronic Traumatic Encephalopathy: A Case Series and Overview on Putative Ethiopathogenetic Mechanisms. Int J Environ Res Public Health. 2021;18. https://doi.org/10.3390/ijerph18030876
- Iverson GL. Suicide and Chronic Traumatic Encephalopathy. J Neuropsychiatry Clin Neurosci. 2016;28:9-16. https://doi.org/10.1176/appi.neuropsych.15070172
- Suter CM, Affleck AJ, Lee M, Pearce AJ, Iles LE, Buckland ME. Chronic traumatic encephalopathy in Australia: the first three years of the Australian Sports Brain Bank. Med J Aust. 2022;216: 530-531. https://doi.org/10.5694/mja2.51420
- Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122-1129. https://doi.org/10.1212/WNL.0b013e3182a55f7f
- Liang C-S, Li D-J, Yang F-C, Tseng P-T, Carvalho AF, Stubbs B, et al. Mortality rates in Alzheimer’s disease and non-Alzheimer’s dementias: a systematic review and meta-analysis. Lancet Healthy Longev. 2021;2: e479-e488. https://doi.org/10.1016/S2666-7568(21)00140-9
- Mackay DF, Russell ER, Stewart K, MacLean JA, Pell JP, Stewart W. Neurodegenerative Disease Mortality among Former Professional Soccer Players. N Engl J Med. 2019;381: 1801-1808. https://doi.org/10.1056/NEJMoa1908483
- Russell ER, Mackay DF, Lyall D, Stewart K, MacLean JA, Robson J, et al. Neurodegenerative disease risk among former international rugby union players. J Neurol Neurosurg Psychiatry. 2022. https://doi.org/10.1136/jnnp-2022-329675
- Taioli E. All causes of mortality in male professional soccer players. Eur J Public Health. 2007;17:600-604. https://doi.org/10.1093/eurpub/ckm035
- Binney ZO, Bachynski KE. Estimating the prevalence at death of CTE neuropathology among professional football players. Neurology. 2019;92:43-45. https://doi.org/10.1212/WNL.0000000000006699
- Thomas E, Fitzgerald M, Cowen G. Does Australia have a concussion “epidemic”? Concussion. 2020;5:CNC70. https://doi.org/10.2217/cnc-2019-0015
- Keene CD, Latimer CS, Steele LM, Mac Donald CL. First confirmed case of chronic traumatic encephalopathy in a professional bull rider. Acta Neuropathol. 2018;135:303-305. https://doi.org/10.1007/s00401-017-1801-z
- Laver K, Cumming RG, Dyer SM, Agar MR, Anstey KJ, Beattie E, et al. Clinical practice guidelines for dementia in Australia. Med J Aust. 2016;204:191-193. https://doi.org/10.5694/mja15.01339
- Moll van Charante EP, Richard E, Eurelings LS, van Dalen J-W, Ligthart SA, van Bussel EF, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388:797-805. https://doi.org/10.1016/S0140-6736(16)30950-

