Abstract
The last 15 years has seen the discovery of treatable antibody-mediated CNS diseases. The accurate detection of specific antibodies helps support patient diagnosis, treatment and predict prognosis. Here we discuss some of the limitations of different antibody detection technologies, caveats in study designs that impacts on the interpretation of assays for routine clinical use and highlight a lack of data on serum versus CSF testing in this new field of neuroimmunology.
Introduction
In the last 15 years, multiple central nervous system disease-associated and likely disease-causing antibodies have been discovered.1-5 These autoantibodies bind the extracellular portion of proteins expressed in the central nervous system and lead to a loss of protein function, typically either by cross-linking and internalisation, or complement-mediated destruction of the cell expressing the antibody target. The detection of these antibodies is important for patient diagnosis, prognosis and management.
Pathogenic antibodies versus biomarkers
Indirect immunohistochemistry (IHC) on frozen tissue sections and immunoprecipitation of radiolabelled brain extracts have been the mainstay of diagnostic laboratories for decades, and have been a part of the process of antibody target discovery.1,6 These methods do not distinguish between antibodies that bind cytosolic or nuclear targets that are considered epiphenomena, but are useful biomarkers often of paraneoplastic disease, and antibodies that bind native, surface-expressed glycoproteins that have pathogenic potential. Biomarker antibodies most often bind denatured protein and are routinely detected by line blot assays, whereas pathogenic antibodies require native three-dimensional structures as their assay substrate. Understanding this important difference has led to the development of new ways to detect these recently-discovered neuronal surface autoantibodies (NSAbs; Figure 1).
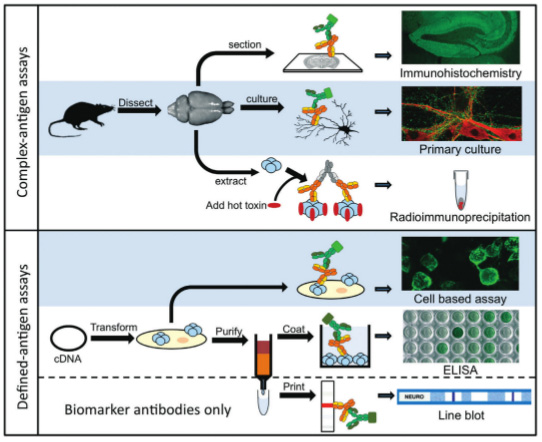
Known NSAb targets include the N-methyl-d-aspartate receptor (NMDAR), aquaporin-4 (AQP4), myelin oligodendrocyte glycoprotein (MOG), leucinerich, glioma-inactivated 1 (LGI1), gamma aminobutyric acid receptor B (GABABR), gamma aminobutyric acid receptor A (GABAAR) and contactin-associated receptor protein 2 (CASPR2).1-3
The more recently developed assays use live primary cultures or transfected mammalian cells as their substrates (highlighted in blue in Figure 1). Primary cultures, which are still predominantly research tools, are used to demonstrate the presence of antibodies in serum or CSF which are likely to impact on live cell function. The cultures are often hippocampal neurons, astrocytes or oligodendrocytes. These are low throughput tests requiring two to three weeks in culture before antibody testing is possible, and are not antigen-specific. In addition, the lack of any architecture in this system can impact on protein expression and antibody binding. For example, astrocytes express low levels of membrane AQP4 in culture, whereas dense arrays of AQP4 are seen on the astrocyte end-feet that abut blood vessels in tissue.
When the antibody target is known, the wide availability of cDNA encoding human genes has enabled the development of antigen-specific cell based assays.2,6 Mammalian cells transiently or stably transfected with a specific target express native protein on the cell surface that is most often detected with fluorescent secondary antibodies. The binding is visualised by microscopy or quantified by flow cytometry. This has become the method of choice in recent years to detect these newly identified pathogenic antibodies, and can be modified for high-throughput.6 Controls include observing serum binding to cells transfected with a different protein or use of untransfected cells to confirm specific binding to the target protein and for quantitative assays determination of cut-offs using healthy control sera are important.
Assessment of assays: developing a paradigm?
CNS diseases associated with NSAbs are important as they often respond to immunotherapies, but they are rare. It is impractical to determine the true sensitivity or specificity of these assays when the prevalence is a few patients per 100,000. Hence, in the field of NSAb detection, the term ‘sensitivity’ is often used to describe the number of positives identified in a cohort of clinically-defined patients. For example, in AQP4-antibody assays, patients with clinically definite NMO are often used to determine assay sensitivity. The most useful controls to define assay specificity are often patients with a clinical overlap that are pathologically different, such as patients with multiple sclerosis in the case of NMO. Be aware that there is a risk of misdiagnosis in patients with clinical overlap. Importantly, double-blinding of assay interpretation should be part of the study design: no antibody information is available to the clinicians and no clinical details are available to the laboratory scientists. This seems a reasonable study set-up,7 but it has caveats.8 The small cohort size, often less than 100 patients per group, means that a few samples can have a large effect on the final assay sensitivity and specificity. This makes it difficult to draw conclusions about the metrics of an assay from different studies even when identical clinical criteria are used to define the patient cohorts (see ref 8 for discussion). To circumvent this issue, the same samples should be tested on different assays for the same antigen. Also, as seen in patients with NMO, there are clinically indistinguishable patients that are likely to be truly seronegative who have different treatment requirements. Separating these patients is an important aspect of diagnostic assays that is subsumed within the clinically-definite cohort in this study design, so not examined.
The dichotomy: sensitivity versus specificity
Which assay is clinically most useful: a highly-specific assay that may miss some samples with perhaps low antibody titres, or a highly-sensitive assay that identifies extra positive samples in the clinically-defined relevant patients (shown as red dots above the cut-off in Figure 2) but which also shows positivity in clinically irrelevant or control individuals (blue dots in Figure 2)?
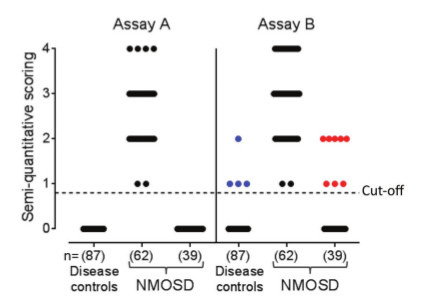
The implication is that the value of the antibody in unrelated diseases can be ignored on clinical grounds, and the increase in sensitivity in the correct clinical context is diagnostically useful. However, it is likely that an assay with ‘false-positive’ results in irrelevant individuals will also have a similar proportion of ‘false-positive’ results in the clinically relevant group. Serum versus CSF for neural antibody testing Should we test serum or CSF? CSF is cleaner than serum containing much less protein and immunoglobulin. It can be tested at higher concentrations, often neat, and produces less background staining which leads to fewer clinically-irrelevant results in NMDAR-antibody assays for example.9 However, the total amount and concentration of antigen-specific antibody is higher in the serum. This provides a more sensitive source of antibodies, and multiple studies have demonstrated that patients with low AQP4 antibody titres in their serum (end-point dilution of 1:250 or less) are not identified in the CSF.10,11 Further research using paired serum and CSF samples is required to determine if the best sample to test will be individual to each assay or will be broadly applicable to all assays for NSAbs. Currently, we and others recommend simultaneous testing of paired serum and CSF samples. Evolution of diagnostic criteria sans antibody testing Identification of disease-specific antibodies has enabled the development of diagnostic criteria. Appreciation of these clinical features has then allowed clinicians to make a diagnosis without antibody results.12,13 This is especially useful either early in the disease course when the results are not yet available, or at centres without access to testing. However, the antibody status often guides prognosis and long-term treatment, so world-wide access to these assays is an important goal. Conclusions Antibody assays are useful tools in the diagnosis and management of patients with treatable antibody-mediated CNS diseases. Future studies should address and optimise paradigms to evaluate NSAb-detection methods.
References
- Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies Ann. N. Y. Acad. Sci. 2015;1338:94-114.
- Lennon VA, Kryzer TJ, Pittock SJ Verkman AS and Hinson SR. IgG marker of optico-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005;202(4):473-7.
- Reindl-M, Di Pauli-F, Rostasy K and Berger T. The spectrum of MOG autoantibody-associated demyelinating disease. Nat. Rev. Neurol. 2013;9(8):455-61.
- Irani SR, Gelfand JM, Al-Diwani A, Vincent A. Cell-surface central nervous system autoantibodies: Clinical relevance and emerging paradigms. Ann Neurol. 2014;10;76(2):168–84.
- Varley J, Vincent A, Irani SR. Clinical and experimental studies of potentially pathogenic brain-directed autoantibodies: current knowledge and future directions. J Neurol. 2015 Apr;262(4):1081–95.
- Waters P, Pettingill P and Lang B. (2016) Detection methods for neural antibodies. Chapter 9: 147-163 in Handbook of Clinical Neurology Eds. Pittock SJ, Vincent A.
- Waters P, McKeon A, Leite MI et al. Serologic diagnosis of NMO: A multicentre comparison of aquaporin-4 IgG assays. 2012;78:665-71.
- Waters P, Pittock SJ, Jarius S et al. Evaluation of aquaporin-4 antibody assays. Clinical and Experimental Neuroimmunology 2014;5(3):290-303.
- Gresa-Arribas N, Titulaer MJ, Torrents A et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167-77.
- Takahashi T, Fujihara K, Nakashima I. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain 2007;130:1235-43.
- Jarius S, Franciotta D, Paul F et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: frequency, origin, and diagnostic relevance. J. Neuroinflammation 2010;7:52.
- Wingerchuk D, Banwell B, Bennett JL et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-89.
- Graus F, Titulaer M, Balu R et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.



