Abstract
Dietary restriction (DR) interventions, which encompass both chronic and intermittent reductions in energy intake, are emerging as potential therapeutic approaches for dampening neuroinflammation and demyelination in multiple sclerosis (MS). Mechanisms mediating the beneficial effects of DR include the regulation of pro- and anti-inflammatory signalling molecules and gut microbiome remodelling. This article summarises the preclinical evidence supporting the role of DR in attenuating disease in animal models of MS and the developing clinical evidence indicating the safety and feasibility of such DR interventions in people with MS (pwMS).
Multiple sclerosis (MS) is considered an autoimmune disease of the central nervous system (CNS), resulting from the complex interplay between genetic and environmental risk factors. Several epidemiological studies conducted over the last decade have established the association between early-life obesity and elevated future risk of MS development [1]. Overweight and obesity (BMI ≥25 or 30, respectively) in adolescents and young adults increased MS risk by two-fold [2-5]. Mendelian randomisation studies have correlated genetic determinants of high BMI with heightened MS susceptibility, accounting for possible confounding lifestyle and socioeconomic factors and supporting a causal relationship between obesity and MS [6-7]. Moreover, obesity was associated with an almost two-fold increased relapse risk, greater annual increase in disability [8], and higher brain volume loss [9].
Multiple mechanisms may underlie the obesity-mediated increase of MS risk, some of which are not clearly understood and are the subject of ongoing investigations. Firstly, obesity is characterised by chronic low-grade inflammation with increased secretion of inflammatory mediators including IL-6, IL-12 and TNF-α and enhanced production of reactive oxygen species by adipose tissue macrophages [10-11]. Furthermore, higher adiposity is associated with increased serum levels of leptin, a pro-inflammatory adipokine [12]. This chronic inflammatory state may act to predispose overweight and obese individuals to the development of autoimmunity. Moreover, obesity is linked to dysbiotic alterations of the gut microbiota [13-14]. The gut microbiota is emerging as a potential modulator of pathogenic immune responses, with animal studies providing evidence for the role of gut microbiota in regulating T lymphocyte differentiation, CNS inflammation and microglia function, as well as myelination and blood-brain barrier integrity [15-18]. Correspondingly, people with MS (pwMS) display moderate gut microbiota dysbiosis [19,20].
Dietary restriction (DR) without malnutrition is a powerful intervention shown to extend healthy lifespan in many animal species, including non-human primates [21]. Furthermore, DR promotes weight loss and reduces multiple markers of inflammation in humans and also the experimental autoimmune encephalomyelitis (EAE) model of MS [22-27]. DR also prevents demyelination and promotes remyelination in toxin-induced models of MS [26,28-30]. In this article, we will review the beneficial effects of DR, including its ability to lower levels of pro-inflammatory molecules and reshape gut microbiome composition, highlighting the potential utility of DR for protecting against neuroinflammation and demyelination in pwMS.
Dietary restriction
Dietary restriction (DR) is defined here as a reduction in total food intake, either chronically or intermittently, whilst maintaining proper nutrition. Chronic DR, also known as calorie restriction (CR), entails a variable reduction in energy intake every day (usually ~20% in humans and up to 40-50% in preclinical models), wherein meal frequency remains unchanged. Intermittent fasting (IF) involves complete abstinence or substantial reduction of energy intake for periods of time, usually 12 hours or longer, and unrestricted feeding during meal times [31]. Examples of IF regimens include time-restricted feeding (TRF), in which total daily food intake is limited to a specific timeframe within the day (typically lasting between 6-8 hours), fasting or drastic caloric reduction (e.g., consumption of 500 calories per day) on alternate days (alternate day fasting or alternate day modified fasting), fasting for 2 days per week (5:2 diet), and fasting mimicking diet (FMD) which comprises several days (usually 5-7 days in humans or 3 days in preclinical models) of drastically reduced calorie intake [32]. Collectively, DR interventions can induce healthy weight loss in overweight individuals and more importantly, exert beneficial anti-inflammatory and neuroprotective effects both in relation to and independent of reduced adiposity [24].
Balancing the pro-inflammatory versus anti-inflammatory cytokine milieu
The adipose tissue serves as not only an energy reservoir, but also an endocrine organ, secreting cytokines and hormones, collectively termed “adipokines”, which can modulate immune responses [33]. A standard Western diet (WD) is characterised by the regular intake of high amounts of processed foods, red meat, high-fat dairy products, high-sugar, and pre-packaged foods and leads to excess adiposity with consequent adipokine dysregulation characterised by upregulation of pro-inflammatory molecules, such as leptin, C-reactive protein, TNFα and IL-6, and downregulation of anti-inflammatory factors, such as adiponectin [34] (Figure 1). CR and IF interventions reduce visceral adiposity in humans [35,36] and restore the balance between pro-inflammatory and anti-inflammatory mediators in animals and humans [22,37,38]. DR reliably decreases leptin levels and increases adiponectin levels in animals and humans [24,39,40] (Figure 1). Pertinently, pwMS display elevated leptin and reduced adiponectin levels, with leptin concentration correlating negatively with the number of regulatory T cells [41,42]. Hence, DR restriction may be an effective tool for tempering the dysregulated cytokine milieu in MS (Figure 1).
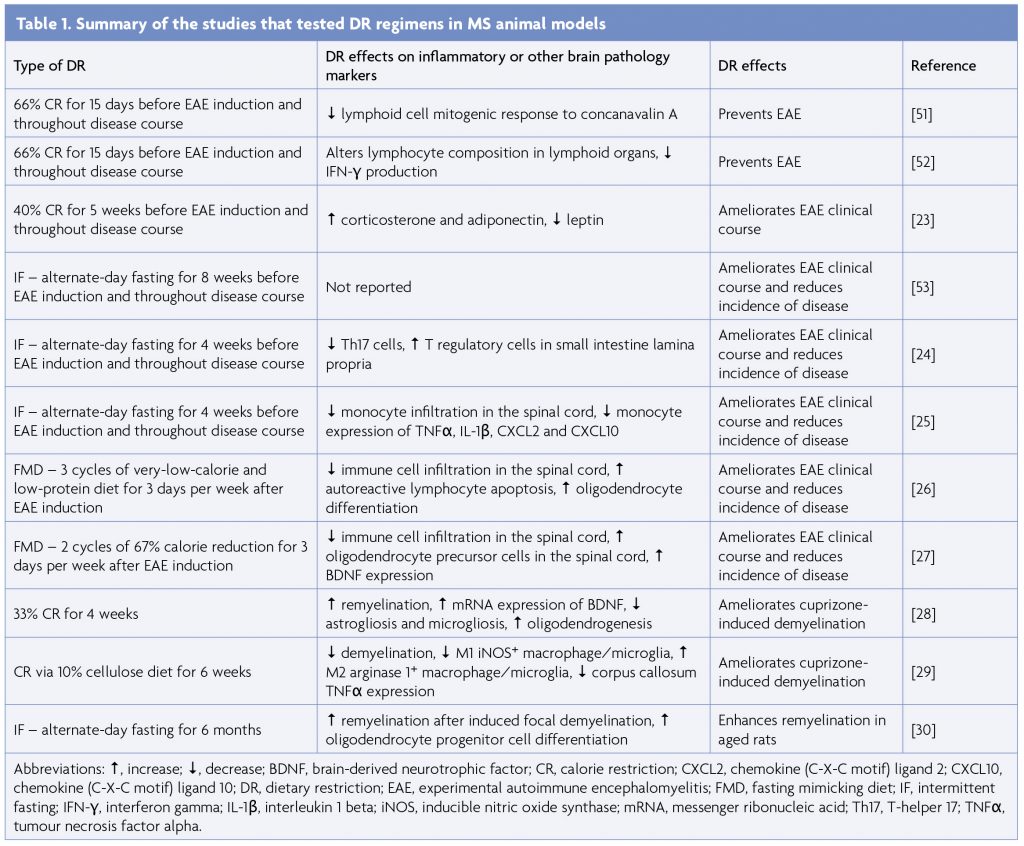
Gut microbiome and neuroinflammation
A considerable number of studies have reported alterations in the gut microbiome composition in pwMS. Unfortunately, there is little overlap in results between studies and only some taxa are consistently reported as differentially represented. Several studies reported an overrepresentation of the genera Akkermansia in pwMS compared to healthy controls [15,19,43] together with a reduced abundance of Bacteroidaceae, Faecalibacterium, Clostridium species and Prevotella strains [44-46]. Both Clostridium and Bacteroidaceae have immunomodulatory effects, promoting the differentiation of murine regulatory T cells and the production of IL-10 [47-49] (Figure 1). It is unclear whether these gut-microbiota alterations in MS are a consequence of the disease or contribute to disease pathogenesis. However, in support of a real pathogenic role is the finding that gut microbiota or gut-derived molecules obtained from pwMS can modulate disease in the main MS animal model, EAE, when transferred into mice [15,43]. Specifically, recipient mice inoculated with microbiota from pwMS showed increased incidence of spontaneous EAE [43] and exacerbated EAE clinical severity with decreased regulatory T cell expression compared to those inoculated with healthy control microbiota [15]. Furthermore, extracts from specific MS-associated bacterial species increased in vitro differentiation of pathogenic CD4+ T helper 1 cells, whilst extracts from bacterial species that were diminished in pwMS boosted regulatory T cell differentiation [15]. These findings indicate a role for MS-related microbiota in shaping immune phenotypes and function.
DR interventions have the potential to positively influence gut microbiota composition. For example, life-long calorie restriction changed gut microbiota structure in mice, as marked by an enrichment of anti-inflammatory bacterial strains including Lactobacilli [50]. Microbial alterations were accompanied by reductions in serum levels of lipopolysaccharide-binding protein, a marker of gut-derived endotoxin load [50], which is also correlated with systemic inflammation.
Search strategy
To find studies testing DR regimens in preclinical models of MS and pwMS, a literature search was conducted in PubMed in September 2021 using the search string ((multiple sclerosis) OR (CNS autoimmunity) OR (experimental autoimmune encephalomyelitis) OR (experimental allergic encephalomyelitis)) AND ((dietary restriction) OR (calorie restriction) OR (caloric restriction) OR (fasting) OR (intermittent)). After reading titles and abstracts, only original research articles investigating restriction of total food intake (not specific macronutrients or micronutrients) were included.
Effects of dietary restriction in preclinical models
There is strong preclinical evidence supporting the efficacy of DR regimens, including both CR and IF, in protecting against neuroinflammation and demyelination (Table 1). The preventative effects of CR in EAE were first demonstrated in 2004 [51]. Prior to immunisation, Lewis rats were subjected to 15 days of severe CR, equivalent to 66% reduction from ad libitum intake and then monitored for EAE symptoms. Whilst 8 out of 9 ad libitum-fed rats developed clinical signs of EAE, calorie-restricted rats did not display any disease manifestations and demonstrated reduced lymphocyte response to T-cell mitogen concanavalin A [51]. A follow-up study utilising the same 15-day CR protocol revealed that moderate CR (33% reduction) was insufficient for preventing EAE progression, whilst severe 66% CR-induced inhibition of EAE development was likely mediated by decreased IFN-γ production [52]. Our group demonstrated that a month of 40% CR in mice delayed EAE clinical onset and decreased disease severity [23]. CR was associated with increased levels of adiponectin and corticosterone, and decreased levels of IL-6 and leptin [23].
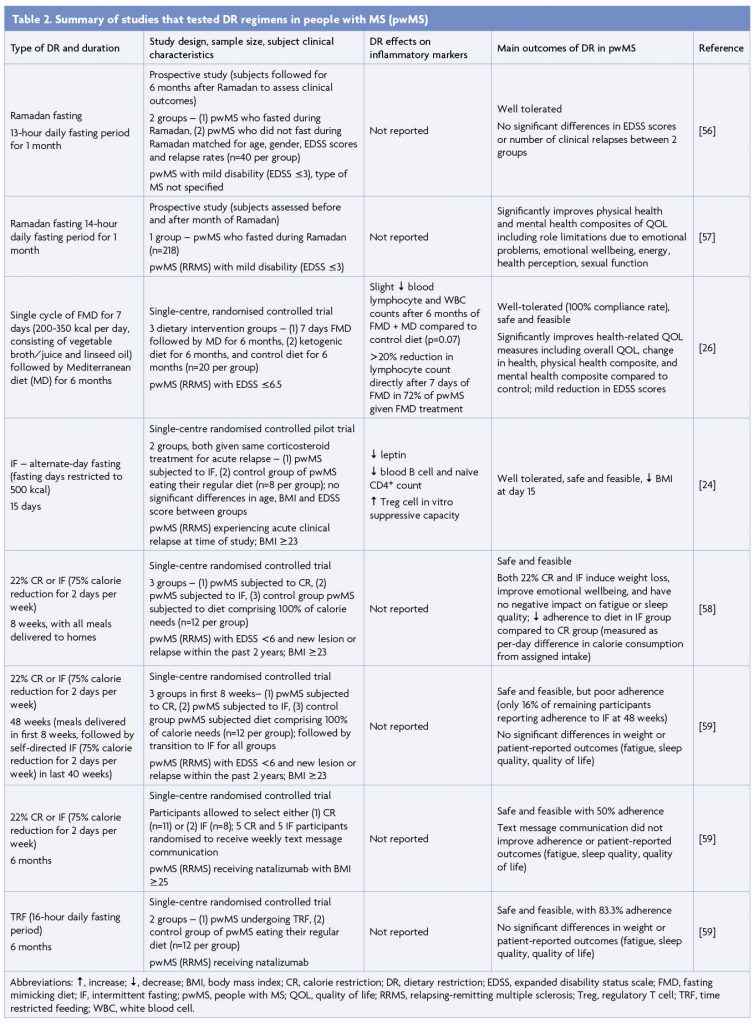
If administered before immunisation and throughout the course of EAE similarly delayed disease onset and reduced the incidence and severity of disease [24,53]. Along with attenuation of EAE, IF decreased Th17 cells and increased regulatory T cells in the small intestine lamina propria and also increased the diversity and altered the composition of the gut microbiome [24]. Notably, gut microbiota transfer from IF mice to naïve EAE recipients recapitulated the protective effects of IF, suggesting the role of microbiota in mediating the favorable effects of IF [24]. Another study found that IF conferred protection against EAE by preventing monocyte recruitment to the CNS and downregulating monocyte expression of pro-inflammatory genes including TNFα, IL-1β, CXCL2 and CXCL10 [25]. Importantly, IF did not compromise the immune response to bacterial infection or tissue injury [25]. FMD, a form of IF applied in cycles which consist of several days of severe reduction in calorie intake followed by ad libitum feeding, has also been effective in ameliorating EAE [26,27]. FMD administered therapeutically after disease onset suppressed autoimmunity as evidenced by reduced pro-inflammatory cytokines, Th1 and Th17 cells and antigen-presenting cells and promoted recovery as demonstrated by enhanced oligodendrocyte regeneration, remyelination [26], and brain-derived neurotrophic factor (BDNF) expression [27].
The beneficial effects of DR have also been reproduced in preclinical models of demyelination and remyelination [26,28-30]. In the cuprizone model (in which oligodendrocyte death is caused by administration of a toxin), 33% DR improved coordination and balance and enhanced corpus callosum remyelination. DR reduced astrogliosis and microgliosis and expanded the oligodendrocyte population [28]. Further, DR skewed macrophage/microglia polarisation towards the anti-inflammatory M2 phenotype in the cuprizone model [29].
Macrophage/microglia phenotypic switching may be modulated by activation of nutrient sensors and modulation of immunometabolic pathways [54,55]. In another study, 6 months of alternate-day fasting increased remyelination in aged rats after focal demyelination induced by ethidium bromide injection through restoring the regenerative capacity of oligodendrocyte precursors [30]. Overall, preclinical data provide promising evidence of the preventative and therapeutic effects of DR in dampening autoimmune and demyelinating responses in experimental models of MS.
Effects of dietary restriction in people with MS
The effects, safety and feasibility of DR in pwMS have been investigated in several studies (Table 2). A 6-month prospective study of 40 pwMS with mild disability (expanded disability status scale-EDSS score ≤3) found that Ramadan fasting, lasting approximately 13 hours daily for a month (however fasting periods can vary between 11 to 18 hours depending on year and geographical location), was safe and did not exacerbate disease [56]. Ramadan fasting (14-hour daily fast) in pwMS with mild disability also significantly boosted physical and mental health measures including energy, health perception and emotional wellbeing [57]. Several interventional DR regimens have been tested in pwMS. A single 7-day cycle of FMD followed by a Mediterranean diet for 6 months improved health-related quality of life metrics and mildly reduced EDSS scores [26]. We compared the effects of 15 days of IF (intake limited to 500 calories every second day) and regular feeding in 16 pwMS undergoing acute MS relapse and receiving corticosteroid treatment [24]. IF was well-tolerated, reduced leptin levels, and recapitulated gut microbiome alterations seen in EAE mice subjected to IF [24]. An 8-week randomised controlled study assessed the effects of 22% CR and IF (2 days of 75% reduction in energy intake and 5 days of ad libitum feeding) in 36 pwMS [58]. Both dietary regimens were concluded to be safe and feasible and were associated with significant improvements in emotional health, whilst adherence was greater in the CR regimen [58]. The same research group performed 6-month pragmatic randomised controlled trials of 22% CR, IF (75% calorie reduction for 2 days per week) and TRF (in which consumption of all daily calories was limited to an 8-hour interval), with feasibility and patient adherence as primary outcome measures [59]. Whilst all DR regimens tested proved to be feasible, self-reported adherence was much higher for the TRF diet than both CR and IF regimens over 6 months, which both demonstrated poor long-term adherence [59]. Altogether, a variety of different DR interventions have proven to be safe and feasible in pwMS.
Presently, there are several ongoing clinical trials of DR in pwMS. To investigate whether the anti-inflammatory and gut microbiome-modulating properties of IF observed in preclinical studies are recapitulated in pwMS, we are currently conducting a 12-week randomised controlled study investigating the effects of IF (2 fasting days/week) on peripheral blood immunological parameters, metabolic profiles and gut microbiota composition in pwMS (NCT03539094). Other ongoing trials are focused on determining whether DR can improve clinical outcomes, which is currently inconclusive. An 18-month, 3-armed study is comparing the occurrence of new cerebral lesions as measured by magnetic resonance imaging between 111 pwMS randomly assigned either a ketogenic diet, an IF regimen consisting of TRF (fasting for 14 hours per day) and an additional 1 week of fasting every 6 months, or a control vegetarian-based diet (NCT03508414). Lastly, a study is investigating the effects of 15-20% calorie restriction, with or without abstinence from dairy and gluten products, on MS progression as well as immune cell activity and metabolism (NCT04042415).
Potential risks of DR
Whilst no serious adverse effects have been reported in clinical trials of DR in pwMS, it is important to be aware of the potential risks that may accompany DR and potential contraindications that may render individuals unsuitable for DR. Mild symptoms experienced by pwMS undergoing DR included fatigue and headaches [26,58,59], although these may or may not be directly related to DR. An assessment of adverse events occurring during medically supervised water-only fasting for ≥2 days consecutively, with a patient cohort not specific to pwMS, described fatigue, insomnia, nausea, headache, hypertension (which was likely incidental due to pre-existing hypertension), presyncope, dyspepsia, and back pain as effects present in more than 25% of visits (from a total of 768 visits), in order of frequency [60]. Contraindications to DR may include low body weight or BMI, pregnancy, very young or old age, comorbidities such as diabetes, and prescription of specific medications. Further, clinical trials have been conducted only in relapsing-remitting MS (RRMS) patients with mild to moderate disability, thus DR may not be appropriate for pwMS with severe disability.
Conclusion and future perspectives
Since the characterisation of obesity as a risk factor for MS, subsequent investigations into the mechanisms underlying this association have implicated the involvement of cytokines from adipose tissue and dysbiotic microbiota in MS. In particular, DR has emerged as an effective method of counteracting the detrimental effects associated with increased adiposity. Indeed, mounting preclinical evidence suggests that DR exerts neuroprotective effects, which are mediated by the modulation of pro- and anti-inflammatory molecules and alterations to the gut microbiome, among other possible mechanisms. Clinical trials have confirmed the safety and feasibility of various DR regimens and demonstrated DR-induced improvements in quality of life measures in pwMS. There is a need for larger and longer randomised controlled studies to produce strong definitive evidence linking DR with improved clinical outcomes, before any clinical recommendations can be made. Long-term patient adherence has been a barrier to determining whether DR can improve clinical disease outcomes and is an important factor to consider when selecting any particular DR regimen. It’s important to recognise that DR alone might not be effective in significantly improving clinical outcomes. However, it can be considered a valuable complementary intervention to commonly used disease modifying treatments and future trials should take into consideration the possibility of an integrated approach. Additionally, the potential risks of DR need to be understood and vulnerable populations of pwMS unsuited to DR regimens need to be identified.
References
- Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13(1):25-36. https://doi.org/10.1038/nrneurol.2016.187
- Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology. 2009;73(19):1543-50. https://doi.org/10.1212/WNL.0b013e3181c0d6e0
- Hedstrom AK, Olsson T, Alfredsson L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler. 2012;18(9):1334-6. https://doi.org/10.1177/1352458512436596
- Wesnes K, Riise T, Casetta I, Drulovic J, Granieri E, Holmoy T, et al. Body size and the risk of multiple sclerosis in Norway and Italy: the EnvIMS study. Mult Scler. 2015;21(4):388-95. https://doi.org/10.1177/1352458514546785
- Gianfrancesco MA, Acuna B, Shen L, Briggs FB, Quach H, Bellesis KH, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obes Res Clin Pract. 2014;8(5):e435-47. https://doi.org/10.1016/j.orcp.2014.01.002
- Gianfrancesco MA, Barcellos LF. Obesity and Multiple Sclerosis Susceptibility: A Review. J Neurol Neuromedicine. 2016;1(7):1-5. https://doi.org/10.29245/2572.942X/2016/7.1064
- Mokry LE, Ross S, Timpson NJ, Sawcer S, Davey Smith G, Richards JB. Obesity and Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med. 2016;13(6):e1002053. https://doi.org/10.1371/journal.pmed.1002053
- Tettey P, Simpson S, Taylor B, Ponsonby AL, Lucas RM, Dwyer T, et al. An adverse lipid profile and increased levels of adiposity significantly predict clinical course after a first demyelinating event. J Neurol Neurosurg Psychiatry. 2017;88(5):395-401. https://doi.org/10.1136/jnnp-2016-315037
- Mowry EM, Azevedo CJ, McCulloch CE, Okuda DT, Lincoln RR, Waubant E, et al. Body mass index, but not vitamin D status, is associated with brain volume change in MS. Neurology. 2018;91(24):e2256-e64. https://doi.org/10.1212/WNL.0000000000006644
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175-84. https://doi.org/10.1172/JCI29881
- Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155(4):407-17. https://doi.org/10.1111/imm.13002
- Procaccini C, Pucino V, Mantzoros CS, Matarese G. Leptin in autoimmune diseases. Metabolism. 2015;64(1):92-104. https://doi.org/10.1016/j.metabol.2014.10.014
- Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26(1):5-11. https://doi.org/10.1097/MOG.0b013e328333d751
- Aron-Wisnewsky J, Prifti E, Belda E, Ichou F, Kayser BD, Dao MC, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68(1):70-82. https://doi.org/10.1136/gutjnl-2018-316103
- Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. 2017;114(40):10713-8. https://doi.org/10.1073/pnas.1711235114
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. https://doi.org/10.1126/scitranslmed.3009759
- Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016;6:e774. https://doi.org/10.1038/tp.2016.42
- Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O’Leary OF. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol Psychiatry. 2015;78(4):e7-9. https://doi.org/10.1016/j.biopsych.2014.12.023
- Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. https://doi.org/10.1038/ncomms12015
- Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:28484. https://doi.org/10.1038/srep28484
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201-4. https://doi.org/10.1126/science.1173635
- Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging (Albany NY). 2016;8(7):1416-31. https://doi.org/10.18632/aging.100994
- Piccio L, Stark JL, Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol. 2008;84(4):940-8. https://doi.org/10.1189/jlb.0208133
- Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell metabolism. 2018;27(6):1222-35 e6. https://doi.org/10.1016/j.cmet.2018.05.006
- Jordan S, Tung N, Casanova-Acebes M, Chang C, Cantoni C, Zhang D, et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell. 2019;178(5):1102-14 e17. https://doi.org/10.1016/j.cell.2019.07.050
- Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016;15(10):2136-46. https://doi.org/10.1016/j.celrep.2016.05.009
- Bai M, Wang Y, Han R, Xu L, Huang M, Zhao J, et al. Intermittent caloric restriction with a modified fasting-mimicking diet ameliorates autoimmunity and promotes recovery in a mouse model of multiple sclerosis. J Nutr Biochem. 2021;87:108493. https://doi.org/10.1016/j.jnutbio.2020.108493
- Mojaverrostami S, Pasbakhsh P, Madadi S, Nekoonam S, Zarini D, Noori L, et al. Calorie restriction promotes remyelination in a Cuprizone-Induced demyelination mouse model of multiple sclerosis. Metab Brain Dis. 2020;35(7):1211-24. https://doi.org/10.1007/s11011-020-00597-0
- Zarini D, Pasbakhsh P, Nekoonam S, Mojaverrostami S, Ghasemi S, Shabani M, et al. Protective Features of Calorie Restriction on Cuprizone-induced Demyelination via Modulating Microglial Phenotype. J Chem Neuroanat. 2021;116:102013. https://doi.org/10.1016/j.jchemneu.2021.102013
- Neumann B, Baror R, Zhao C, Segel M, Dietmann S, Rawji KS, et al. Metformin Restores CNS Remyelination Capacity by Rejuvenating Aged Stem Cells. Cell Stem Cell. 2019;25(4):473-85 e8. https://doi.org/10.1016/j.stem.2019.08.015
- Longo VD, Panda S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016;23(6):1048-59. https://doi.org/10.1016/j.cmet.2016.06.001
- Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG, 3rd, et al. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity (Silver Spring). 2018;26(2):254-68. https://doi.org/10.1002/oby.22065
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010-3. https://doi.org/10.2337/db06-1656
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85-97. https://doi.org/10.1038/nri2921
- Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61(9):943-50. https://doi.org/10.1093/gerona/61.9.943
- Faris MAE, Jahrami HA, Alhayki FA, Alkhawaja NA, Ali AM, Aljeeb SH, et al. Effect of diurnal fasting on sleep during Ramadan: a systematic review and meta-analysis. Sleep Breath. 2020;24(2):771-82. https://doi.org/10.1007/s11325-019-01986-1
- Willette AA, Coe CL, Birdsill AC, Bendlin BB, Colman RJ, Alexander AL, et al. Interleukin-8 and interleukin-10, brain volume and microstructure, and the influence of calorie restriction in old rhesus macaques. Age (Dordr). 2013;35(6):2215-27. https://doi.org/10.1007/s11357-013-9518-y
- Spezani R, da Silva RR, Martins FF, de Souza Marinho T, Aguila MB, Mandarim-de-Lacerda CA. Intermittent fasting, adipokines, insulin sensitivity, and hypothalamic neuropeptides in a dietary overload with high-fat or high-fructose diet in mice. J Nutr Biochem. 2020;83:108419. https://doi.org/10.1016/j.jnutbio.2020.108419
- Higami Y, Pugh TD, Page GP, Allison DB, Prolla TA, Weindruch R. Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. FASEB J. 2004;18(2):415-7. https://doi.org/10.1096/fj.03-0678fje
- Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordr). 2010;32(1):97-108. https://doi.org/10.1007/s11357-009-9118-z
- Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, et al. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2005;102(14):5150-5. https://doi.org/10.1073/pnas.0408995102
- Kraszula L, Jasinska A, Eusebio M, Kuna P, Glabinski A, Pietruczuk M. Evaluation of the relationship between leptin, resistin, adiponectin and natural regulatory T cells in relapsing-remitting multiple sclerosis. Neurol Neurochir Pol. 2012;46(1):22-8. https://doi.org/10.5114/ninp.2012.27211
- Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017;114(40):10719-24. https://doi.org/10.1073/pnas.1711233114
- Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med. 2015;63(5):729-34. https://doi.org/10.1097/JIM.000000000`0000192
- Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv. 2017;3(7):e1700492. https://doi.org/10.1126/sciadv.1700492
- Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One. 2015;10(9):e0137429. https://doi.org/10.1371/journal.pone.0137429
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337-41. https://doi.org/10.1126/science.1198469
- Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3(5):487-95. https://doi.org/10.1038/mi.2010.29
- Ramakrishna C, Kujawski M, Chu H, Li L, Mazmanian SK, Cantin EM. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat Commun. 2019;10(1):2153. https://doi.org/10.1038/s41467-019-09884-6
- Zhang C, Li S, Yang L, Huang P, Li W, Wang S, et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun. 2013;4:2163. https://doi.org/10.1038/ncomms3163
- Esquifino AI, Cano P, Jimenez V, Cutrera RA, Cardinali DP. Experimental allergic encephalomyelitis in male Lewis rats subjected to calorie restriction. J Physiol Biochem. 2004;60(4):245-52. https://doi.org/10.1007/BF03167069
- Esquifino AI, Cano P, Jimenez-Ortega V, Fernandez-Mateos MP, Cardinali DP. Immune response after experimental allergic encephalomyelitis in rats subjected to calorie restriction. J Neuroinflammation. 2007;4:6. https://doi.org/10.1186/1742-2094-4-6
- Kafami L, Raza M, Razavi A, Mirshafiey A, Movahedian M, Khorramizadeh MR. Intermittent feeding attenuates clinical course of experimental autoimmune encephalomyelitis in C57BL/6 mice. Avicenna J Med Biotechnol. 2010;2(1):47-52.
- Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. https://doi.org/10.3389/fimmu.2014.00614
- Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J Immunol. 2017;198(3):1006-14. https://doi.org/10.4049/jimmunol.1601515
- Saadatnia M, Etemadifar M, Fatehi F, Ashtari F, Shaygannejad V, Chitsaz A, et al. Short-term effects of prolonged fasting on multiple sclerosis. Eur Neurol. 2009;61(4):230-2. https://doi.org/10.1159/000197108
- Etemadifar M, Sayahi F, Alroughani R, Toghianifar N, Akbari M, Nasr Z. Effects of prolonged fasting on fatigue and quality of life in patients with multiple sclerosis. Neurol Sci. 2016;37(6):929-33. https://doi.org/10.1007/s10072-016-2518-9
- Fitzgerald KC, Vizthum D, Henry-Barron B, Schweitzer A, Cassard SD, Kossoff E, et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult Scler Relat Disord. 2018;23:33-9. https://doi.org/10.1016/j.msard.2018.05.002
- Roman SN, Fitzgerald KC, Beier M, Mowry EM. Safety and feasibility of various fasting-mimicking diets among people with multiple sclerosis. Mult Scler Relat Disord. 2020;42:102149. https://doi.org/10.1016/j.msard.2020.102149
- Finnell JS, Saul BC, Goldhamer AC, Myers TR. Is fasting safe? A chart review of adverse events during medically supervised, water-only fasting. BMC Complement Altern Med. 2018;18(1):67. https://doi.org/10.1186/s12906-018-2136-6


