Summary
- Fatigue experienced following ABI is multifactorial and difficult to measure
- Fatigue impacts on rehabilitation, levels of social participation and quality of life
- There is a growing evidence base around fatigue, but this remains limited regarding management
- This clinical model may support development of a shared understanding, guide intervention and reduce vulnerability to fatigue for individuals
- More research into both the subjective (experienced, reported) and objective (physiological and neuropsychological) aspects of fatigue, and their interplay, is required
Fatigue is one of the most commonly reported, distressing, and persistent symptoms after acquired brain injury (ABI), including traumatic head injury and stroke, with an estimated incidence of more than 60% across the range of injury severity [1,2]. Injury severity is not necessarily a predictive factor in the severity of fatigue experienced, with fatigue reported after mild and very severe ABI [3]. Persistent fatigue is associated with lower rates of return to work [4] and higher mortality post-stroke [5]. Despite this, evidence for management remains inadequate [6] and clinically people report feeling unprepared for this consequence of their brain injury.
Defining and therefore operationalising fatigue is challenging as there are many confounding factors associated with it. It is now widely accepted as a multidimensional, biopsychosocial construct, authors describing both primary and secondary, or physiological (central and peripheral) fatigue and psychological fatigue impacting resultant behaviour, felt experience and its presentation within societal and cultural contexts [2]. Central fatigue is considered to result from impairment to structures within the central nervous system and is characterised by depletion of hormones and neurotransmitters. Peripheral fatigue is considered as a diminished ability to contract muscles, involving the peripheral motor and sensory systems [2,7]. Brain structures and networks thought to be involved include the hypothalamic-pituitary axis, ascending reticular activating system, frontal cortex and basal ganglia. For example, neural circuits involved in the regulation of attention and executive function may contribute to the development of tiredness and aversion to effort leading to fatigue [8], whilst other authors [9] note involvement of the ventro-medial pre-frontal cortex following penetrating traumatic brain injury.
Confounding factors contributing towards fatigue following brain injury incorporate pathophysiological, physical, mood, and cognitive elements, including slowed speed of processing and difficulty sustaining attention [10], executive dysfunction [11], reward and effort perception [9], anxiety and depression [12,13], sleep disturbance [12] and pain [14,15]. Clinically these interacting elements may be considered as ‘vulnerability factors’ for fatigue as they are common consequences of an acquired brain injury and so addressing these factors may lead to a reduction in fatigue experienced and enhance levels of social participation.
Many people experience fatigue as a consequence of participating in everyday activities. Pathological fatigue, which may indicate the need for clinical intervention, does not necessarily dissipate with rest and is of greater intensity and duration compared to ‘normal fatigue’ experienced following exertion, with a corresponding impact on the ability to undertake functional activities. People experiencing pathological fatigue following ABI frequently refer to their brain as “shutting off”, with an intolerance to sensory stimuli and struggle to think and communicate effectively. ‘Mental’ fatigue (as opposed to peripheral fatigue) is frequently described as unpleasant and people perceive a lack of control over it with a negative impact on their level of self-efficacy [16]. Cantor and colleagues [6] suggest a ‘coping hypothesis’ with fatigue experienced considered a response to reduced cognitive functioning and tasks requiring more effort. They consider fatigue after brain injury as an “umbrella term” describing “different symptom clusters with potentially heterogeneous aetiologies and consequences” [6] [p. 880]. Patients report that fatigue significantly impacts their ability to participate in rehabilitation and daily living activities and influences their mood, relationships and quality of life. Eilertsen, Ormstad and Kirkevold [17] identified the need for acknowledgement of this distressing symptom from others as a key factor influencing coping as it presented as a ‘hidden dysfunction’ that could be misinterpreted by others.
There are numerous self-report fatigue scales available, though few valid and reliable measures have been developed for people with ABI. Such scales include the Barrow Neurological Institute Fatigue Scale [18] for acute stages post-injury, the Mental Fatigue Scale [19] which has been developed for the ABI population, the Neurological Fatigue Index – Stroke [20] which has been developed for Stroke. As a consequence, many clinicians and researchers make use of scales initially developed for other diagnostic groups, such as the modified Fatigue Impact Scale [21] and the Fatigue Severity Scale [22]. Additionally subscales of more generic ABI symptom questionnaires may indicate the presence of clinically significant fatigue such as the Rasch-analysed EBIQ subscale [23] or the Profile of Mood States [24].
Scales available may address different aspects of fatigue (e.g. intensity, severity, characteristics, and impact on activities of daily living) over different timeframes. Therefore from a clinical rehabilitation perspective, measures are selected based upon the clinical question to be addressed or the domain which is expected to be changed as a result of the intervention. In our experience, when people begin to feel less fatigued, they naturally attempt to engage in more activity and so their overall level of fatigue may not reduce significantly, as measured on a fatigue scale. However, it is possible to capture changes in their felt experience, such as a reduction in the level of worry about their fatigue, an increase in their sense of control or self-efficacy, an increase in their perceived quality of life or an increase in their awareness and understanding of fatigue. This change can be captured through using a recognised scale of these constructs or for example using an individualised likert scale before and after intervention.
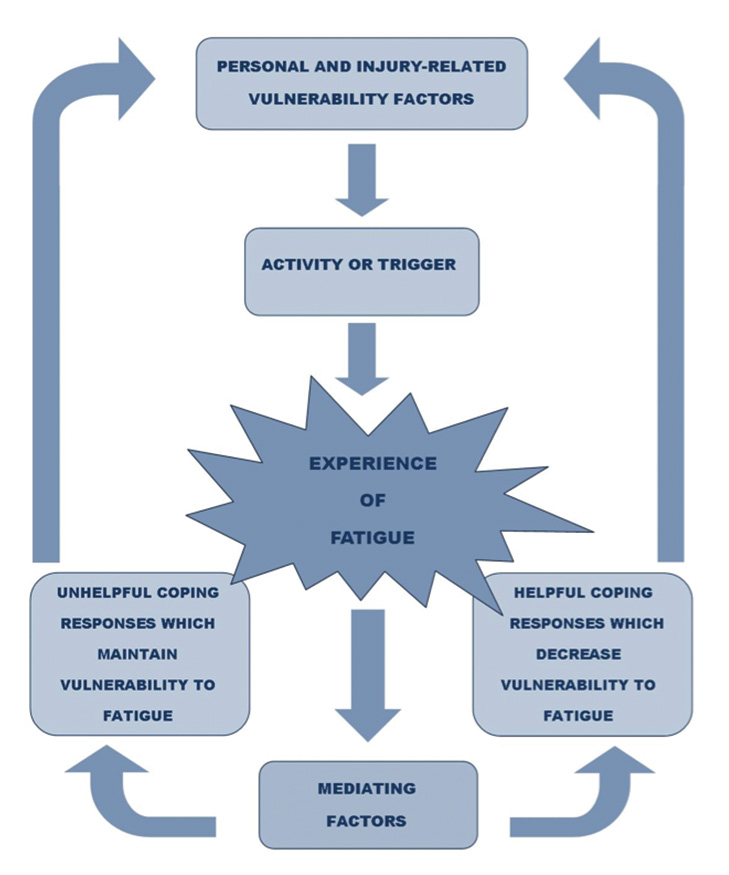
In terms of clinical management, given that fatigue is considered a multidimensional construct, attention should be paid to the variety of factors that may contribute to both performance fatigability (objective signs) and perception of fatigue (subjective symptoms). This involves identifying and addressing both personal and injury-related factors (primary causes and secondary consequences) that make an individual vulnerable to fatigue following ABI. Awareness of indicators of fatigue for that individual, mediating factors affecting behaviour (e.g. what they know about management, what they are doing, and context) and potential triggers need to be considered in order to understand how an individual may respond to fatigue and support them to develop more helpful coping strategies. Fatigue management aims to increase a person’s ability to participate in their desired activities more effectively, improve their quality of life and improve their sense of control over their fatigue.
There is an acknowledged discrepancy between objective signs (performance fatigability) and subjective experience (perception) of fatigue in the literature, which has led to a proposal for a unified taxonomy to guide assessment and intervention [25]. Several models of fatigue have been proposed in the literature. However, to date, none of these have been found to be clinically useful for understanding fatigue following acquired brain injury, to capture all aspects of this challenging construct and an individual’s potential responses to it. The following model has therefore been developed by our clinical team, inspired by the fatigue model proposed for multiple sclerosis [cited in 26], current evidence, and clinical experience, and it has been found useful when working with people with fatigue following ABI.
The clinical model proposed provides guidance on domains of functioning to assess and support fatigue management. A review of personal factors, including coping styles and co-morbid illness, is recommended, with evaluation of injury-related vulnerability factors that could be contributing to fatigue based on pathology and assessment of associated physical, cognitive and psychological factors. This may indicate medical referral if physiological or psychiatric conditions are suspected which require further assessment and intervention e.g. endocrine dysfunction.
In terms of identifying triggers for fatigue, it is recommended to support the individual, and/or their significant other, to keep a ‘fatigue diary’ by monitoring changes in levels of fatigue before and after engagement in certain activities. By operationalising changes in energy levels, this could potentially enable assignment of ‘points’ to different activities to support pacing, identifying those activities or situations which ‘drain’ the resources and those which may ‘recharge the body/mind’ to support participation throughout the day. Use of analogies in fatigue management, such as recharging a phone battery, can be helpful. One important aspect of clinical intervention for people with ABI is to notice signs and symptoms of fatigue before they perceive their brain as ‘shutting down’ or fully ‘draining their battery’. Self-monitoring of fatigue levels can be challenging following ABI secondary to dysexecutive syndrome, or as a consequence of reduced interoception. Identifying personal signs and symptoms of fatigue, through discussion, observation and asking others for signs of fatigue they notice will enable creation of a personalised ‘fatigue scale’ to indicate signs and symptoms of fatigue at an early enough stage to take action.
Neuropsychological formulation and multidisciplinary assessment can then support identification of current coping responses (helpful and unhelpful) and mediating factors influencing choice of coping, which may include knowledge and awareness of fatigue and management strategies, the context, beliefs and preferred coping styles. Unhelpful coping responses may include a ‘boom and bust’ approach, avoidance of activity, and overuse of stimulants such as coffee or energy drinks. Education about fatigue has been demonstrated as an effective intervention via group [27,28] and/or individual intervention for people with Stroke and ABI. Mindfulness-Based Stress Reduction has also been demonstrated as effective when delivered as an eight-week group programme [29]. Sinclair and colleagues [30] have identified short wave (blue) light therapy as a potentially useful intervention. Cognitive and environmental strategies and mood management all contribute towards reducing the effort involved in completing activities and associated errors, which may then contribute towards reducing rumination and self-criticism. Adequate hydration, nutrition, and physical exercise implementing good sleep hygiene, and having an understanding of preferences and challenges in sensory processing will also aid fatigue management depending on the vulnerability factors identified. The use of behavioural experiments to test out the impact of coping strategies and beliefs about the self has been useful in fatigue management intervention within our neuropsychological rehabilitation setting. It is recommended to identify helpful coping responses to both reduce the effort involved and to re-energise oneself, both ‘in the moment’ and ‘in anticipation’ of certain triggers when planning to support an individual to pace themselves. Through creation of a personalised fatigue formulation and management plan, based on the proposed clinical model, a shared understanding and validation of the fatigue experience can be facilitated.
Assessment and management of fatigue remain complex and challenging for both clinicians and researchers. A clinically useful model to aid a shared understanding and response to fatigue and thereby reduce an individual’s vulnerability to fatigue is proposed. This model, developed by the clinical team at the Oliver Zangwill Centre for Neuropsychological Rehabilitation (http://www.ozc.nhs.uk), seems to provide a helpful tool to support management advice and is based upon current evidence available. Further research is required to operationalise and validate fatigue assessment tools and to identify specific interventions that may reduce an individual’s vulnerability to fatigue following ABI. Given the multiple factors and interventions that may be involved, a specialist neurological multidisciplinary rehabilitation team are likely best placed to support people with fatigue following ABI.
References
- Parks NE, Eskes GA, Gubitz GJ, Reidy Y, Christian C, Phillips SJ. Fatigue Impact Scale demonstrates greater fatigue in younger stroke survivors. The Canadian Journal of Neurological Sciences 2012;39(5):619-25. https://doi.org/10.1017/S0317167100015353
- Ponsford J, Ziino C, Parcell DL, Shekleton JA, Roper M, Redman JR, Phipps-Nelson J, Rajaratnam SMW. Fatigue & Sleep Disturbance Following Traumatic Brain Injury – Their Nature, Causes, and Potential Treatments. Journal of Head Trauma Rehabilitation. 2012;27(3):224-33. https://doi.org/10.1097/HTR.0b013e31824ee1a8
- Olver JH, Ponsford JL & Curran CA. Outcome following traumatic brain injury: a comparison between 2 and 5 years after injury. Brain Injury 1996;10(11):841-8. https://doi.org/10.1080/026990596123945
- Andersen G, Christensen D, Kirkevold M, and Johnsen SP. Post‐stroke fatigue and return to work: a 2‐year follow‐up. Acta Neurologica Scandinavica 2012;125(4):248-53. https://doi.org/10.1111/j.1600-0404.2011.01557.x
- Naess H, Lunde L, Brogger J, Waje-Andreassen U. Fatigue among stroke patients on long-term follow-up. The Bergen Stroke Study. Journal of the Neurological Sciences 2012;312(1-2):138-41. https://doi.org/10.1016/j.jns.2011.08.002
- Cantor JB, Gordon W, Gumber S. What is post TBI fatigue? NeuroRehabilitation 2013; 32(4):875-83. https://doi.org/10.3233/NRE-130912
- Elovic EP, Dobrovic NM & Fellus JL. Fatigue after Traumatic Brain Injury. In: DeLuca J (Ed) Fatigue as a Window to the Brain. 2005; London: The MIT Press. Ch.6:91-105.
- Kutlubaev MA, Mead GE. One step closer to understanding post-stroke fatigue. Neurology 2012;79(14):1414-15. https://doi.org/10.1212/WNL.0b013e31826d604e
- Pardini M, Krueger F, Raymont V, Grafman J. Ventromedial prefrontal cortex modulates fatigue after penetrating traumatic brain injury. Neurology 2010;74(9):749-54. https://doi.org/10.1212/WNL.0b013e3181d25b6b
- Ronnback L & Johansson B. Long-lasting mental fatigue after traumatic brain injury or stroke – a new perspective. 2012; Saarbrucken, Germany: Lambert Academic Publishing.
- Juengst S, Skidmore E, Arenth PM, Niyonkuru C, Raina KD. Unique Contribution of Fatigue to Disability in Community-Dwelling Adults with Traumatic Brain Injury. Archives of Physical Medicine and Rehabilitation 2013;94:74-9. https://doi.org/10.1016/j.apmr.2012.07.025
- Schnieders J, Willemsen D, De Boer H. Factors contributing to chronic fatigue after traumatic brain injury. Journal of Head Trauma Rehabilitation 2012;27(6):404-12. https://doi.org/10.1097/HTR.0b013e3182306341
- Crosby, Gail A, et al. Fatigue after stroke: frequency and effect on daily life. Disability and rehabilitation 2012;34(8):633-7. https://doi.org/10.3109/09638288.2011.613517
- Hoang CLN, Sall JY, Mandigout S, Hamonet J, Macian-Montor F, Daviet J-C. Physical factors associated with fatigue after stroke: An exploratory study. Topics in Stroke Rehabilitation 2012;19(5):369-76. https://doi.org/10.1310/tsr1905-369
- Miller KK, Combs SA, Van Puymbroeck M, Altenburger PA, Kean J, Dierks TA, Schmid AA. Fatigue and pain: Relationships with physical performance and patient beliefs after stroke. Topics in Stroke Rehabilitation 2013;20(4):347-55.
- Muina-Lopez R, Guidon M. Impact of post-stroke fatigue on self-efficacy and functional ability. European Journal of Physiotherapy 2013;15(2):86-92. https://doi.org/10.3109/21679169.2013.792868
- Eilertsen G, Ormstad H, Kirkevold M. Experiences of post-stroke fatigue: Qualitative meta-synthesis. Journal of Advanced Nursing 2013;69(3):514-25. https://doi.org/10.1111/jan.12002
- Borgaro SR, Gierok S, Caples H, Kwasnica C. Fatigue after brain injury: Initial reliability study of the BNI Fatigue Scale. Brain Injury 2004;18(7):685-90. https://doi.org/10.1080/02699050310001646080
- Johansson B, Starmark A, Berglund P, Rodholm M, Ronnback L. A self-assessment questionnaire for mental fatigue and related symptoms after neurological disorders and injuries. Brain Injury 2010;24:2-12 https://doi.org/10.3109/02699050903452961
- Mills RJ, Pallant JF, Koufali M, Sharma A, Day S, Tennant A, Young CA. Validation of the Neurological Fatigue Index for stroke (NFI-Stroke). Health and Quality of Life Outcomes 2012; vol. 10 https://doi.org/10.1186/1477-7525-10-51
- Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clinical Infectious Diseases 1994; 18(Supplement 1):S79-83. https://doi.org/10.1093/clinids/18.Supplement_1.S79
- Krupp LB, La Rocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 1989; 46:1121-3. https://doi.org/10.1001/archneur.1989.00520460115022
- Bateman A, Teasdale TW, Willmes K. Assessing construct validity of the self-rating version of the European Brain Injury Questionnaire (EBIQ) using Rasch analysis. Neuropsychological Rehabilitation 2009;19(6):941-54. https://doi.org/10.1080/09602010903021170
- McNair DM, Lorr M & Dropplemann LF. Manual: Profile of Mood States, Revised 1992; SanDiego, CA: Educational and Industrial Testing Service.
- Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013;80(4):409-16. https://doi.org/10.1212/WNL.0b013e31827f07be
- Harrison S. Fatigue Management for people with Multiple Sclerosis (Second Edition) 2007; London: College of Occupational Therapists Specialist Section Neurological Practice.
- Clarke A, Barker-Collo SL, Feigin VL. Post-stroke fatigue: does group education make a difference? A randomized pilot trial. Topics in stroke rehabilitation 2012;19(1):32-9. https://doi.org/10.1310/tsr1901-32
- Cooper J, Reynolds F, Bateman A. An evaluation of a fatigue management intervention for people with acquired brain injury: an exploratory study. British Journal of Occupational Therapy 2009;72(4):174-9. https://doi.org/10.1177/030802260907200407
- Johansson B, Bjuhr H, Ronnback L. Mindfulness-based stress reduction (MBSR) improves long-term mental fatigue after stroke or traumatic brain injury. Brain Injury 2012;26(1314):1621-8. https://doi.org/10.3109/02699052.2012.700082
- Sinclair KL, Ponsford JL, Taffe J, Lockley S W, Rajaratnam SM. Randomized Controlled Trial of Light Therapy for Fatigue Following Traumatic Brain Injury. Neurorehabilitation and neural repair 2013; 1545968313508472. https://doi.org/10.1177/1545968313508472


