Key points
- Fatigue is a common and significantly disabling symptom in MS and its aetiology is poorly understood
- Pharmaceutical trials so far have not had a strong hypothesis in terms of underlying mechanisms. Consequently, the results are disappointing, Amantadine being the only medication currently recommended by the NICE guidelines
- Emerging theoretical research based on homeostatic and interoceptive circuits and potentially supported by neuroimaging may improve our understanding
Abstract
This review aims to inform clinicians about the current strategies in managing fatigue in multiple sclerosis (MS) and the challenges which arise from the insufficient understanding of the underlying mechanisms. It gives an overview of the research undergone to find symptomatic treatment, the non-pharmacological approaches and the scarce knowledge about the impact of disease modifying treatment on fatigue in MS. Finally, it presents a few recent hypotheses and underlines the importance of finding a framework which brings together knowledge in the field.
Background
Fatigue is a common symptom for people with multiple sclerosis (MS), affecting between 65 and 97%,1 independent of physical disability.2 It has a high impact on the quality of life (QoL) affecting productivity and employment.3 However, the causes remain elusive. Issues include the difficulty in fully defining the term and the varied additional factors that can influence it. A consistent measure of fatigue in MS is difficult because there are many types of fatigue, many confounding factors and some of the disease modifying treatments might influence fatigue levels.
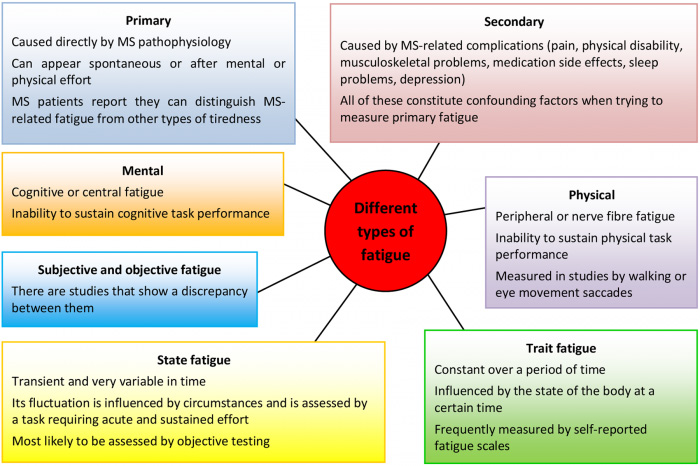
Fatigue is a subjective feeling; therefore, its self-reported nature makes any conclusion based on such results subjective and possibly less reliable. Patients, physicians and researchers use distinct terminologies. There is no clear definition in the literature. To serve accuracy, different types of fatigue in MS have been proposed (Figure 1).
The search for symptomatic medications in MS fatigue
Starting in the 1980s, there have been several trials4 trying to find an efficient treatment for MS fatigue. As the mechanisms underlying it are so poorly understood, there was no clear hypothesis. Only one drug, Amantadine (found to have low to moderate benefit) is recommended by the NICE guidelines.5,6,7 The mechanism by which it alleviates MS fatigue is not entirely clear, but might involve its dopaminergic action.8
After conflicting results in research studies,9,10,11,12,13 Modafinil is not currently recommended for the treatment of MS fatigue.
Modafinil is licenced in the UK for treatment of narcolepsy with or without cataplexy. In 2011, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) evaluated benefits and risks of using Modafinil. In addition, risks associated with Modafinil (including psychiatric disorders, cardiovascular symptoms, and serious skin and multi-organ hypersensitivity reactions) led NICE to conclude that it was not recommended for the treatment of fatigue associated with MS.5
Other medications were studied, with negative results, including: Pemoline, 4-aminopyridine (not effective in cognitive fatigue), L-carnitine and paroxetine.4 Further, the NICE guidelines specifically advise not to give medications such as vitamin B12 intramuscular injections, which have been used in the past, as it was not felt to have a sufficient evidence base.5
The search for non-pharmacological interventions for MS fatigue
A few non-pharmacological approaches have been shown to be effective and are recommended by the current NICE guidelines, such as mindfulness-based training, cognitive-behavioural therapy, fatigue management interventions and physical exercise (aerobic, balance, stretching, including yoga).5
The study with the best methodological quality on mindfulness-based training for MS fatigue was done by Grossman and colleagues.14 Fatigue was found to be significantly reduced post-intervention.14
Probably the most well-known community intervention is the FACETS (Fatigue: Applying Cognitive Behavioural and Energy effectiveness Techniques to lifeStyle) intervention.15 It has been tested in a randomised controlled trial comparing with standard care and it encompassed: education about fatigue in MS, structured resting, pacing, breathing exercises, setting goals and challenging negative thoughts.16 164 patients were randomised and primary outcome data was available for 146. A significant benefit was found after six weekly sessions of 90 minutes. The benefit was still present at one and four months and one year post-intervention.
The role of exercise in alleviating fatigue in MS has been studied, with conflicting results. A recent Cochrane review concluded that exercise has some benefit in MS fatigue17 although we should be aware of the methodological flaws of the existent studies so far.
Although the results of these interventions are encouraging, the main concern is the lack of an active control group and the difficulty in blinding for these sorts of trials. Therefore, the benefit might be related to the attention and input received from professionals, rather than attributed to the interventions per se.
The influence of disease modifying treatment on fatigue
As there have not been randomised controlled trials looking into fatigue as a primary outcome, there is no clear consensus. The subjectivity of measurement is also a possible limitation.
It is well known that MS patients treated with interferon-beta can experience fatigue as a side effect.18 However, interferon-beta has also been reported to have slow to moderate efficacy in alleviating fatigue in MS.19,20 Glatiramer acetate was also reported to reduce fatigue in MS.21 There have been some reports on the superiority of glatiramer acetate over interferon-beta in improving MS fatigue.22,23
There has been some evidence of natalizumab benefit on fatigue.24,25,26 A cross-sectional case-control study found natalizumab more efficient in reducing fatigue comparing to interferon-beta and glatiramer acetate.27 There is however a small study which found no changes with natalizumab in fatigue levels in MS patients.28
There is no evidence for teriflunomide29 or dimethyl-fumarate to improve MS fatigue.30 There is some limited evidence for effectiveness of fingolimod in lowering MS fatigue.31 Also, switching from interferon-beta (but not glatiramer acetate) to fingolimod might reduce fatigue.32 A Cochrane review of alemtuzumab in MS did not find any studies looking into its effects on fatigue.33 There is a need for the available disease modifying treatments to be assessed, from the fatigue point of view.
Additional studies are needed, particularly as the number of disease modifying therapies used in MS has increased, to assess if fatigue can improve with immunotherapies used in the disease. In addition, the apparent difficulty in understanding of the mechanisms underlying MS fatigue has contributed to the conflicting results seen. Future research needs to propose clear and testable hypotheses. It is likely that only with a robust hypothesis, will we be able to develop more effective treatment.
Approaches to understand MS fatigue
These challenges have encouraged research about fatigue in MS from different perspectives. Firstly, the molecular aspects were studied. A relationship was found between elevated blood pro-inflammatory cytokines and increased feelings of fatigue in MS. These results suggested that fatigue might be a form of sickness behaviour.1 However, the studies of inflammatory markers from the blood provided limited information because, due to the existence of the blood-brain barrier, markers in the blood do not necessarily reflect the presence of cytokines in the brain.
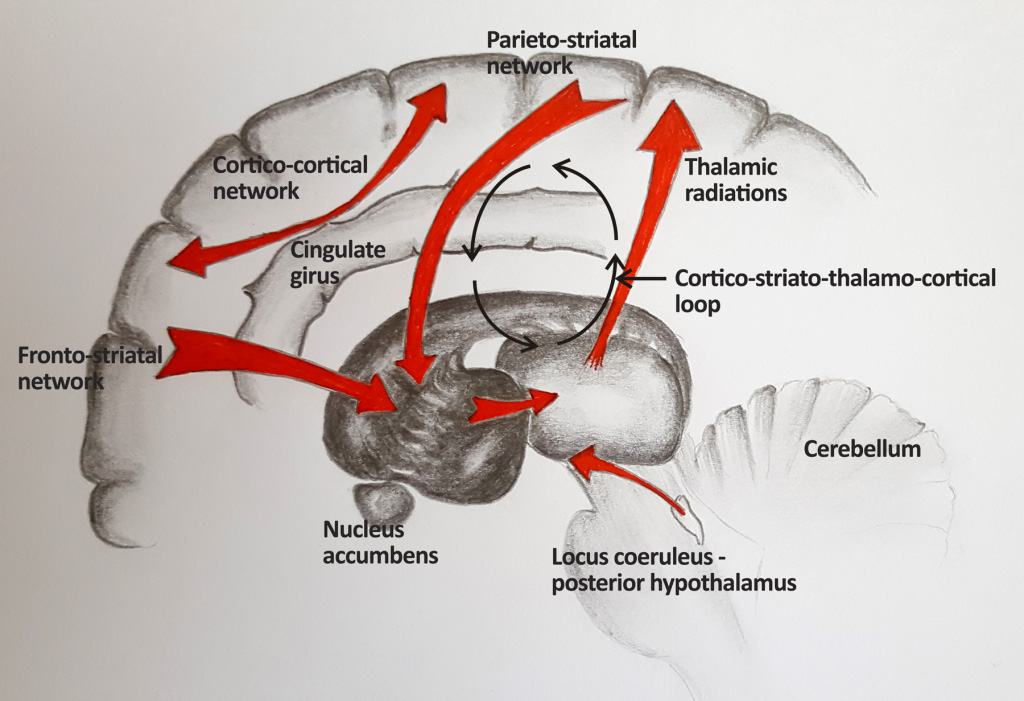
In order to get a better understanding of the exact processes, structural and functional neuroimaging studies were performed. A series of brain areas were found to be statistically correlated to MS fatigue:34 fronto-striatal network, parieto-striatal network, deep grey matter, cortico-cortical networks, cortico-striato-thalamo-cortical loop, fibres connecting locus coeruleus and posterior hypothalamus, cerebellum, cingulate cortex, right anterior thalamic radiations (Figure 2).
Although the areas identified with imaging studies give a potential anatomical rationale for fatigue, the lack of a strong a priori hypothesis to reflect the areas of the brain involved in MS fatigue makes the findings difficult to interpret.
Unifying model for fatigue
A new model is necessary to explain how information is transmitted in the brain in people with MS experiencing fatigue. In order to build such a model, we need to remember that fatigue is a subjective feeling (present in other diseases also). Understanding the way that feelings arise is key to understanding fatigue.35 Hence a framework on how the information related to multiple sclerosis fatigue is processed within the brain is important.
Stephan et al36 propose fatigue to be a feeling arisen from a state of chronic dyshomeostasis. In order for the body to function normally, it needs to inform the brain about its inner state. The information goes up (via interoceptive afferent pathways) to the brain areas that have set points for normal function (hypothalamus, brainstem, spinal cord). These areas receive information about the inner body and check if the values are in the required range. They send further information (viscero-sensory input) to the brain areas that integrate information about the inner body (the viscero-sensory areas).
However, the brain does not only react to the input it receives (homeostasis). It anticipates homeostatic perturbation by making predictions. It gives commands and then changes its prior beliefs according to what input it receives back (allostasis). This means that the brain has certain expectations about what is going to happen.36 Although it involves peripheral fatigue, the information on fatigue from sports medicine is within the same line of evidence: the central Governor Model states that human exercise performance is not limited by a failure of homeostasis but is rather regulated in advance in order to avoid it.37
The brain evaluates the accuracy of its own predictions by measuring the degree of surprise present when input comes back to inform it. In an acute setting (for example an acute feeling of fatigue), the brain is surprised about what information it is receiving. This results in an adaptive reaction- seeking resting behaviour, therefore consuming less energy. However, persistent interoceptive surprise represents a warning that the brain cannot control perturbations (chronic dyshomeostasis). Moreover, the brain has a metacognitive layer, represented by its beliefs about its own capacity and performance. In the case of chronic dyshomeostasis, the metacognitive layer receives the message that there is a low capacity of the brain to control the inner body states (higher order beliefs about lack of control).36 Stephan et al raised the hypothesis that the feeling of discomfort related to fatigue could be related to metacognitive dysfunction induced by chronic dyshomeostasis.36
Fatigue in multiple sclerosis
Several groups have found structures involved in the dopaminergic networks to be related to MS fatigue. The findings supported the model proposed by Chaudhuri and Behan, who view fatigue as a failure of the non-motor functions of the basal ganglia.38 The findings of dysfunction in areas in the brain related to the reward system have led to the development of a dopamine imbalance theory.8 This can be interpreted in light of what we know about the brain: its goal is to be informed if homeostasis is maintained or not. Behaviourally, this is done by seeking pleasure and avoiding pain. Even very simple organisms have seeking and avoidance behaviours (dictated by rules encrypted in their genome), in order to keep the homeostatic levels. In more complex organisms, this developed into the reward and punishment system (the motivation system).
Focusing on fatigue as a feeling, Hanken and colleagues1 proposed the hypothesis that fatigue is a subjective feeling related to inflammation. They described fatigue as a type of inflammation-induced sickness behaviour induced by cytokine-mediated activity in the brain areas involved in interoception and homeostasis. Their theory is that inflammation may redirect the focus to the interoceptive pathway, away from vigilance and attention.
The idea that the interoceptive system is involved in the feeling of fatigue is not restricted to multiple sclerosis. Kadota et al studied patients with post-infectious fatigue and found a sensitisation in the neuro-visceral regulatory circuits, which led to abnormally heightened perceptions of sensations from the body (referred to as physiological hyper-vigilance).39
Conclusions
There is a definite need to find effective medication for MS fatigue. Recent research has linked knowledge from immunology, neuroimaging and consciousness neuroscience, showing that inflammation leads to psychological, emotional and behavioural disturbances. The inflammatory state represents a condition out of the normal homeostatic parameters which is signalled by the afferent interoceptive pathways from which the brain makes hierarchic representations of this bodily state, the highest level being the metacognitive level, which may have a role in the feeling of MS fatigue. Dopaminergic pathways are also involved in MS fatigue and seem to have an important role. Finding a framework that integrates the findings so far and guides further research is crucial to understanding MS fatigue and therefore developing therapeutic targets for it.
References
- Hanken K, Eling P, Hildebrandt H. The representation of inflammatory signals in the brain – a model for subjective fatigue in multiple sclerosis. Front Neurol. 2014;5:264. doi:10.3389/fneur.2014.00264.
- Alvarenga-Filho H, Papais-Alvarenga RM, Carvalho SR, Clemente HN, Vasconcelos CC, Dias RM. Does fatigue occur in MS patients without disability? Int J Neurosci. 2015;125(2):107-115. doi:10.3109/00207454.2014.909415.
- Flesner G, Ek A-C, Landtblom A-M, Söderhamn O. Fatigue in relation to perceived health: people with multiple sclerosis compared with people in the general population. Scand J Caring Sci. 2008;22(3):391-400. doi:10.1111/j.1471-6712.2007.00542.x.
- Tur C. Fatigue Management in Multiple Sclerosis. Curr Treat Options Neurol. 2016;18(6):26. doi:10.1007/s11940-016-0411-8.
- NICE Clinical Guidance 186. Management of multiple sclerosis in primary and secondary care. https://www.nice.org.uk/guidance/cg186. Published 2014.
- Ledinek AH, Sajko MC, Rot U. Evaluating the effects of amantadin, modafinil and acetyl-L-carnitine on fatigue in multiple sclerosis–result of a pilot randomized, blind study. Clin Neurol Neurosurg. December 2013:S86-9. doi:10.1016/j.clineuro.2013.09.029.
- A randomized controlled trial of amantadine in fatigue associated with multiple sclerosis. The Canadian MS Research Group. Can J Neurol Sci. 1987;14(3):273-278.
- Dobryakova E, Genova HM, DeLuca J, Wylie GR. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol. 2015;6:52. doi:10.3389/fneur.2015.00052.
- Rammohan KW, Rosenberg JH, Lynn DJ, Blumenfeld AM, Pollak CP, Nagaraja HN. Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study. J Neurol Neurosurg Psychiatry. 2002;72(2):179-183.
- Stankoff B, Waubant E, Confavreux C, et al. Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology. 2005;64(7):1139-1143. doi:10.1212/01.WNL.0000158272.27070.6A.
- Lange R, Volkmer M, Heesen C, Liepert J. Modafinil effects in multiple sclerosis patients with fatigue. J Neurol. 2009;256(4):645-650. doi:10.1007/s00415-009-0152-7.
- Möller F, Poettgen J, Broemel F, Neuhaus A, Daumer M, Heesen C. HAGIL (Hamburg Vigil Study): a randomized placebo-controlled double-blind study with modafinil for treatment of fatigue in patients with multiple sclerosis. Mult Scler J. 2011;17(8):1002-1009. doi:10.1177/1352458511402410.
- Ford-Johnson L, DeLuca J, Zhang J, Elovic E, Lengenfelder J, Chiaravalloti ND. Cognitive effects of modafinil in patients with multiple sclerosis: A clinical trial. Rehabil Psychol. 2016;61(1):82-91. doi:10.1037/a0039919.
- Grossman P, Kappos L, Gensicke H, D’Souza M, Mohr DC, Penner IK SC. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149. doi:10.1212/WNL.0b013e3181f4d80d.
- Thomas S, Thomas PW, Kersten P, et al. A pragmatic parallel arm multi-centre randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(10):1092-1099. doi:10.1136/jnnp-2012-303816.
- Thomas S, Thomas PW, Nock A, et al. Development and preliminary evaluation of a cognitive behavioural approach to fatigue management in people with multiple sclerosis. Patient Educ Couns. 2010;78(2):240-249. doi:10.1016/j.pec.2009.07.001.
- Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev. 2015;(9):CD009956. doi:10.1002/14651858.CD009956.pub2.


