Hydocephalus is defined and the mechanisms of CSF hydrodynamics discussed. Supplementary tests used in the investigation of idiopathic normal pressure hydrocephalus are reviewed with a detailed explanation of constant flow CSF infusion tests. The principles governing valve selection are illustrated.
Hydrocephalus is the abnormal accumulation of CSF within the cranium due to defective CSF production, flow or absorption. The CSF usually accumulates within the ventricular system, however, ‘external hydrocephalus’ with the widening of the subarachnoid spaces is described. Hydrocephalus can be due to obstructive causes preventing normal CSF flow through the CSF pathways, or due to abnormal absorption of CSF: communicating hydrocephalus. CSF flow studies frequently show a complex picture with contributions from both mechanisms. Overproduction of CSF that exceeds the absorption capacity of the arachnoid granulations is rare.
CSF physiology
CSF is mainly produced by passive ultrafiltration of plasma with some active electrolyte transport from the ventricular choroid plexus. The rate of CSF production is around 20ml/hour, although MRI evidence suggests that this may increase significantly during sleep. To maintain equilibrium, CSF is normally absorbed into the major venous sinuses by a passive mechanism through the one-way valves of the arachnoid villi. The normal CSF pressure at the reference level (the foramen of Monro) in the recumbent adult is 100-200mm H2O (7-15mmHg) with mean pressures of 20mmHg regarded as elevated. Pressures from 0-7mmHg do not usually signify any pathology. The CSF pressure fluctuates with the arterial pulse wave and respiratory excursions.
Symptomatic patients with obstructive hydrocephalus continue producing CSF. CT and MRI scans identify the site of obstruction. The definitive treatment for most of these patients is removal of the obstructive cause. If CSF diversion is required and the outlet of the IIIrd or IVth ventricle obstructed, a IIIrd ventriculostomy is usually the first choice treatment, particularly in newly diagnosed patients with aqueduct stenosis.
Communicating hydrocephalus occurs when the lateral, IIIrd and IVth ventricles appear to communicate freely. The absorption capacity of the arachnoid villi is exceeded or obstruction of CSF flow occurs within the subarachnoid space. This condition is usually managed with a ventriculoperitoneal shunt system and provides the focus for this paper.
Normal pressure hydrocephalus
In the pre-CT scan era Adams et al. reported three cases of ventriculomegaly associated with gait disturbance, dementia and incontinence [1]. All three patients had normal CSF pressure (140-50, 160 and 175mmH2O) on lumbar puncture but improved with either a ventriculo-atrial shunt (two cases) or a Torkildsen (lateral ventricle to cisterna magna) diversion (one case). Two cases were idiopathic and one due to a cyst in the IIIrd ventricle. The condition is now classified as (i) Primary or Idiopathic Normal Pressure Hydrocephalus (INPH) and (ii) Secondary Normal Pressure Hydrocephalus. In the latter group of patients a well-established cause is evident (eg. subarachnoid haemorrhage, traumatic brain injury, meningitis). Whilst the primary pathology may increase the certainty of diagnosing hydrocephalus, the results of treatment may be confounded by the original brain insult.
Even in the presence of a classic triad of symptoms the response to treatment is often disappointing. Indeed Black reported that 67.2% of patients with gait, cognitive and urinary symptoms and signs improved with a shunt [2]. The outcome was significantly worse in patients with only dementia and gait disturbance (31.6% improved). Overall, 35.4% of the 62 patients studied suffered complications, including subdural haematomas and fits. The challenge therefore lies in increasing diagnostic accuracy and timely management at a point when symptoms and signs are retrievable.
Clinical feature of INPH
The symptomatic triad
The gait disturbance in INPH includes at least two of the following features: wide based stance, out-turned feet, decreased step height, decreased step length, decreased speed, increased trunk sway, en bloc turning requiring three or more steps for 180o, and poor heel-toe walking. Cognitive features are wide-ranging and include attention deficits, psychomotor retardation, impaired recall and memory deficits, executive dysfunction, behavioural and personality changes. Such features can be quantified using a summative mental state examination. Urinary dysfunction is characterised by nocturia, urgency, frequency or incontinence reflecting a low capacity neurogenic bladder.
Evidence-based clinical diagnostic criteria for the diagnosis of INPH have only recently been developed. A consensus panel recommends that INPH candidates be categorised into ‘probable’ and ‘possible’ groups based upon history, examination, brain imaging and CSF opening pressure [3].
Probable INPH
This requires a gait disturbance and either cognitive and/or urinary disturbances in a patient over 40 years old. In addition the history, imaging and lumbar puncture opening pressures must be consistent with the diagnosis. The imaging findings are characterised by ventriculomegaly not due to atrophy or obstructive hydrocephalus, associated with one or more of the following; temporal horn enlargement, a callosal angle of 40o or more (due to bowing of the corpus callosum), periventricular lucency not due to ischaemia and a flow void in the aqueduct or IVth ventricle. The accepted range of CSF opening pressure for probable INPH is 70-245mmH2O (5-18mmHg).
Possible INPH
This group may have a more acute history in a younger patient with only one of the triad of symptoms and an opening CSF pressure outside the guidelines above. The imaging findings may appear to be consistent with atrophy.
Imaging
CT and MRI scans provide useful information that helps support a diagnosis of normal pressure hydrocephalus. Ventriculomegaly is an essential finding (Evans ratio >0.3; Widest inter-frontal horn distance / Internal skull distance at level of frontal horn). Other findings such as large temporal horns and sulcal obliteration are inconsistent. Although periventricular and deep white matter lesions are commonly observed in T2-weighted MRI they are also associated with hypertension and cerebrovascular disease and are therefore not pathognomic of hydrocephalus [4]. Post-shunting MRI scans do show an improvement in the frontal horn periventricular changes but such pre-operative features are not required to predict a good outcome [5]. Calculation of the ‘stroke volume’ of CSF moving in a craniocaudal direction during systole using phase contrast CSF velocity MR imaging has shown that a volume greater than 42µL correlated with a favourable response to shunting [6]. This is characterised by a signal flow void. PET cerebral blood flow (CBF) studies have shown that pre and post-shunt assessments of haemodynamic reserve using a carbonic anhydrase inhibitor to stimulate increased PaCO2 indicate that shunt responders show an improvement in their cerebrovascular reserve compared with non-responders [7]. This suggests that altered CBF dynamics are important in the pathogenesis of INPH and in determining the success of treatment. Unfortunately, specific thresholds for low CBF have not been identified as pre-operative predictors of treatment success.
Due to the diagnostic conundrum and the difficulties in determining shunt responsive cases several other tests have been developed to aid management. These include intracranial pressure monitoring, CSF infusion tests, the tap test and a period of CSF drainage. The main drawback of these tests is the low sensitivity and poor predictive value of some tests (see Table 1). The additional tests that are often used are detailed below.
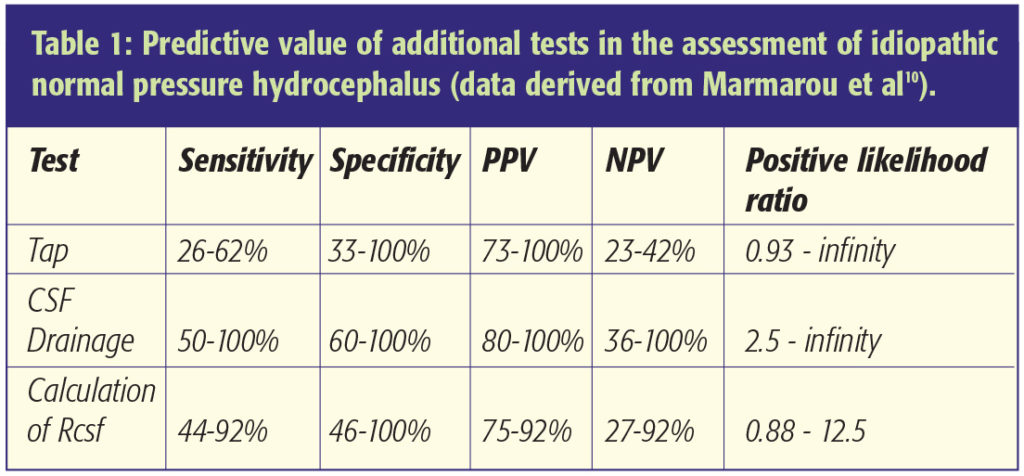
ICP monitoring
Patients with INPH frequently have normal ICP. However, 24 hour monitoring may reveal several abnormalities that indicate poor cerebral compliance (Figure 1). An ICP recording shows systolic and diastolic pulsations. Plateau (A) waves with elevations exceeding 50mmHg for periods of 5-20 min are not normally seen in patients with idiopathic hydrocephalus. However, careful analysis of the ICP trace – using computer software with threshold filters – reveals low amplitude (commonly 1-5mmHg) superimposed B waves with a period of 30 seconds to 2 minutes [8]. The prevalence of B waves appears to increase during normal REM sleep and with rises in intracranial pressure. A recent detailed analysis in patients with communicating and non-communicating hydrocephalus indicates that B waves are commonly observed but have a poor correlation to clinical outcome [9].
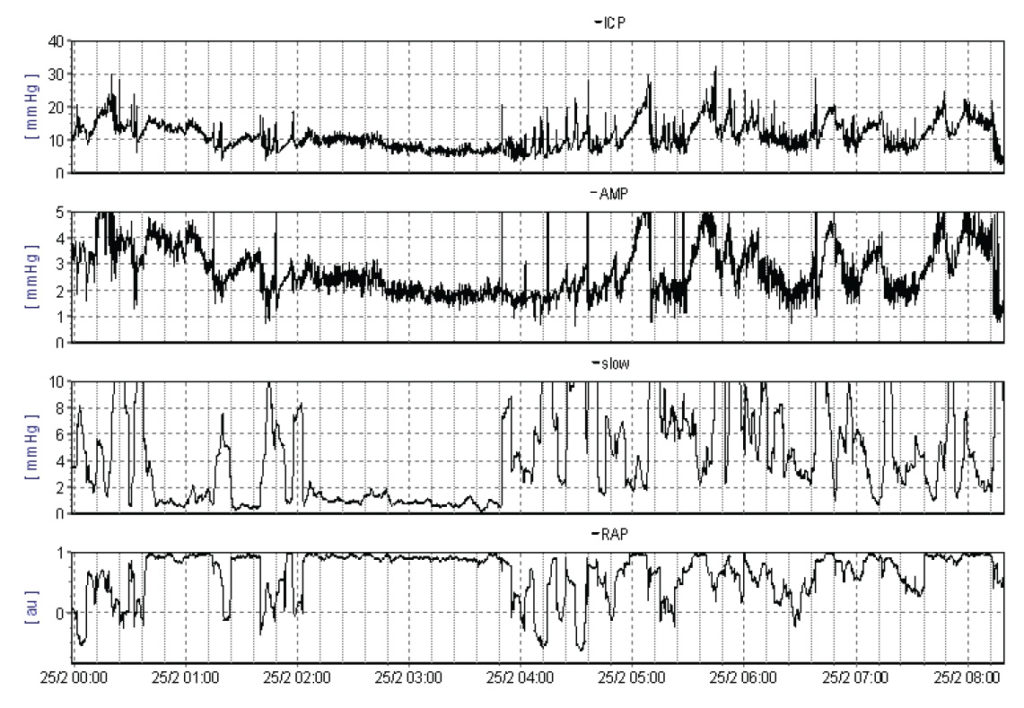
Tap test
Many authors have reported the withdrawal of 40-50ml of CSF as a useful test, with responders benefiting from shunt insertion. However, the test has a low sensitivity (26-62%) and should not be used to rule out a diagnosis of idiopathic normal pressure hydrocephalus [10].
External Lumbar CSF Drainage
This test developed from the concept that a trial of controlled CSF removal (10ml/hr) for 72 hours might predict shunt responders. The sensitivity of the test has been reported as 50-100% with a specificity of 60-80% and a positive predictive value of 80-100% [10].
CSF infusion test
The resistance to CSF absorption by the arachnoid villi can be measured and helps predict shunt responsive patients.
The test is commonly performed in the left lateral position using a constant infusion technique. Lumbar puncture needles are inserted at 2 levels; the use of a solitary needle with a three-way tap is not as reliable. A pressure transducer is connected to one needle and the baseline opening pressure recorded. Normal saline is infused at 1.5ml/min through the second needle whilst the pressure is continuously measured. In most patients the pressure rises steadily and then reaches a plateau. The resistance (Rcsf) to CSF absorption can be calculated using an Ohm’s Law analogy:
Rcsf = Plateau pressure (mmHg) – Baseline pressure (mmHg)
Infusion rate (ml/min)
The Rcsf in normal subjects ranges from 6 to 10mmHg/ml/min. It increases in the elderly. In such cases the rate of CSF production probably decreases to prevent hydrocephalus ensuing [11].
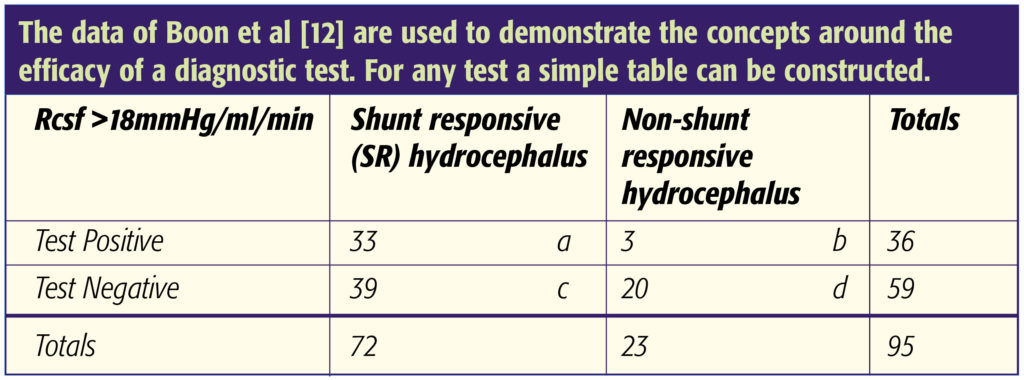
By using an infusion rate of 1.5ml/min a 30-mmHg increase in CSF pressure provides evidence of the Rcsf exceeding 20mmHg/ml/min. The use of higher infusion rates (eg. 3ml/min) imposes limitations in that the pressure needs to rise by 60mmHg to confirm an Rcsf of 20mmHg/ml/min. We recommend aborting the test if CSF pressure exceeds 50mmHg. In this case a minimum value for the Rcsf can still be calculated using the (peak pressure – baseline pressure) as the numerator in the equation. Boon et al. have reported that in patients with probable NPH (mainly idiopathic but also including some secondary cases) a positive response to shunting was likely if Rcsf exceeded 18mmHg/ml/min with a PPV of 92% and a likelihood ratio of 3.5 in their series of 95 patients. However, the sensitivity of the test at this threshold was only 46% although the specificity was high at 87% [12].
Performing the infusion test via a frontal ventricular access device appears to minimise the effect of CSF leakage around lumbar needles and may increase the predictive value of the investigation (Figures 2A and 2B). With sophisticated computer analysis (see http://www.neurosurg.cam.ac.uk/icmplus) of the pressure waveform further information about the elastance and compliance of the craniospinal axis, including the Pressure Volume Index (PVI), can be derived both in lumbar and ventricular CSF infusion studies. This may assist the decision making process in borderline cases.
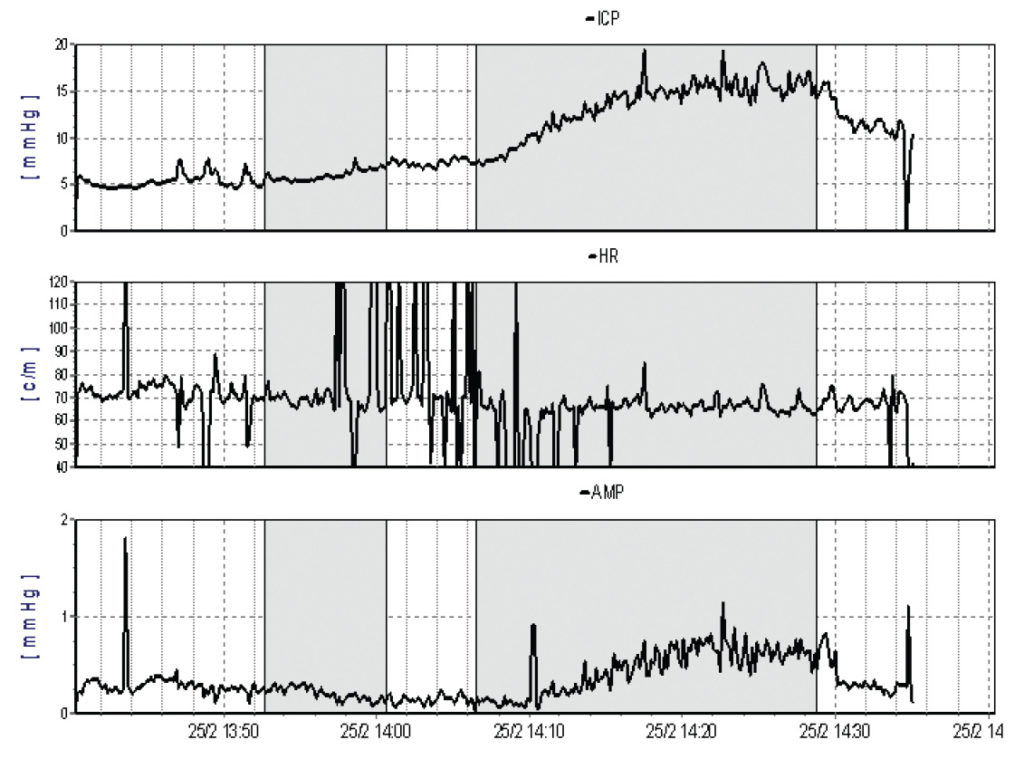
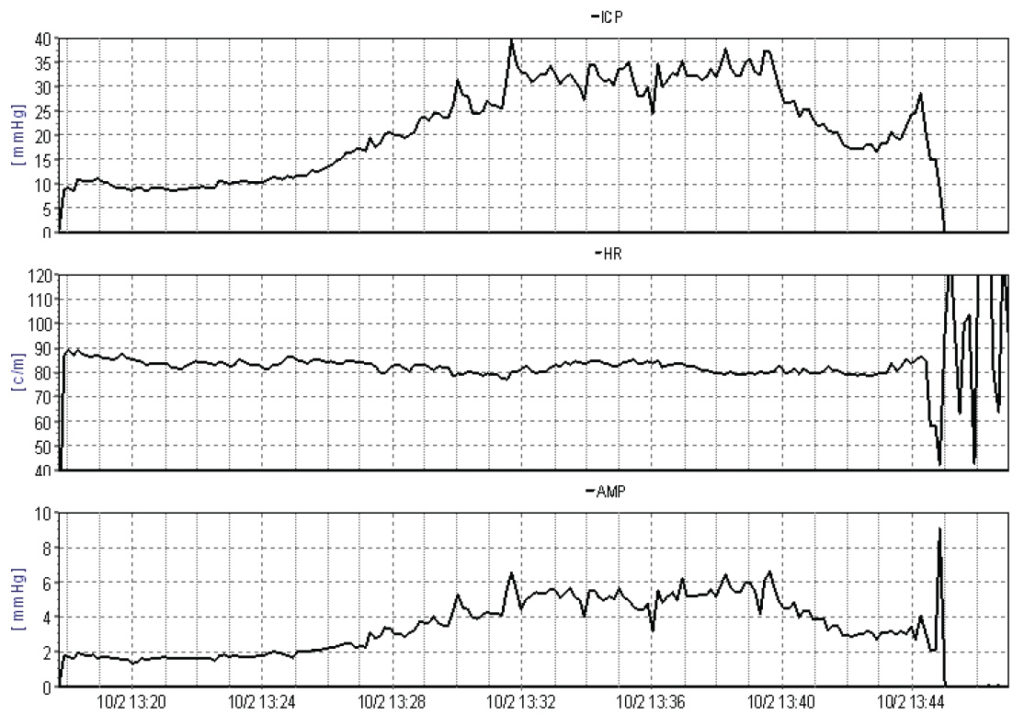
Choosing a CSF shunt
Most neurosurgeons advocate a ventriculo-peritoneal shunt (VP shunt) as the preferred system for implantation. In some circumstances (eg. inadequate absorption in a patient with multiple previous abdominal operations) alternative sites are required (eg. ventriculo-pleural, ventriculo-atrial). VP shunt insertion is associated with numerous potential complications. These include:
• Peri-operative intracranial bleeding
• Infection
• Shunt blockage
- Proximal catheter
- Valve
- Distal
• Shunt component disconnection or migration
• CSF over-drainage - Low pressure headaches
- Intracranial haematomas (usually subdural)
• CSF under-drainage
Attention to detail during the placement of a VP shunt is crucial to minimise the risks of shunt insertion. Meticulous sterility, accurate catheter placement and secure connections between shunt components are essential. Consideration needs to be applied to the choice of shunt hardware. The ventricular catheter most widely used is a straight non-flanged device with multiple apertures in proximity to the catheter tip. There is no consensus over the best anatomical site for catheter placement. The distal catheter provides a conduit to drain CSF to the peritoneal cavity. Distal slit valves are unnecessary and may increase the risk of distal obstruction provided a valve is utilised proximally. Antimicrobial impregnated ventricular and peritoneal catheters have been developed in an attempt to reduce shunt infection rates.
Valves
Valve systems with different hydrodynamic properties have been developed to try and minimise complications such as over-drainage with low-pressure postural headaches and subdural fluid collections. The properties of valves have been independently evaluated in vivo [13]. Valves are designed to be (1) flow regulated or (2) differential pressure regulated (Figure 3). The Orbis Sigma Valve is the archetypal flow controlled device. CSF flows though a diaphragmatic aperture whose diameter decreases as the flow rate rises above 20ml/hr. This increases the resistance of the valve, regulating flow. A safety mechanism leading to a reduction of resistance at differential pressures of 25-30mmHg is incorporated to avoid acute severe elevations in intracranial pressure.
Most valves are differential pressure regulated. These devices are designed to open if the pressure differential across the valve exceeds a set value. However, as the pressure across the valve increases the resistance decreases allowing as much CSF to flow as is required to reduce the ICP near to the opening pressure. Postural over-drainage symptoms are common and may be averted to some extent using anti-siphon devices to minimise flow in the erect posture. Pressure-regulated valves that can be reset using transcutaneous programming devices to higher or lower pressure differentials according to clinical need are now implanted as a matter of routine [14]. Clinical studies, including data from the UK Shunt Registry, do not show significant differences in the shunt revision rate between different valve systems, although the management of complications such as subdural haematomas or hygromas is more readily managed using adjustable valves.
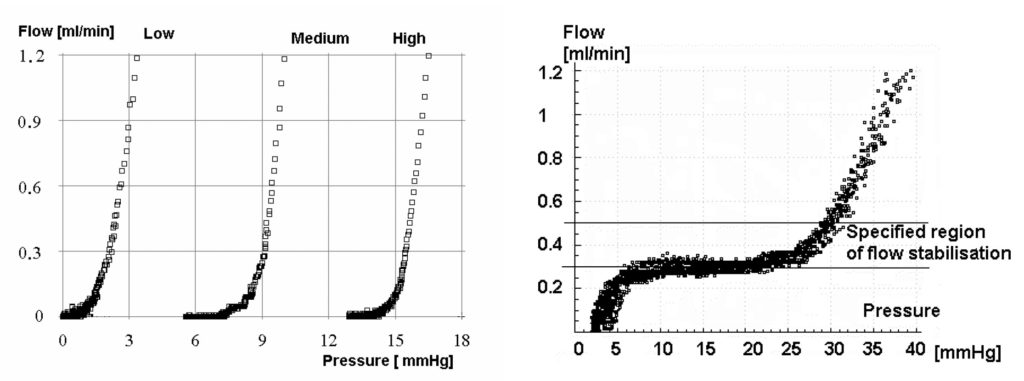
Figure 3B (right): Flow-pressure curve for a flow regulated valve. The flow regulator attempts to change its resistance in response to the differential pressure thereby maintaining flow at a constant level. The Orbis Sigma Valve has a variable resistance that increases in the mid-range acting as a flow control mechanism.
Outcome
The primary pathological process (eg. subarachnoid haemorrhage, head injury) is an important determinant of outcome for patients with secondary hydrocephalus. In patients with idiopathic NPH, comparisons between studies are confounded by the range of symptoms exhibited in this elderly group of patients with significant co-morbidity. Certainly a widely used validated outcome assessment tool would help compare outcomes between studies. In addition, the diagnostic difficulties and variable treatment thresholds between centres influence outcome. The duration of follow-up is also important, with a reported decrease in clinical improvement at five years compared with one year. Improvements in gait decreased from 76% to 47%, improvements in memory disturbances decreased from 48% to 38%, and improvements in urinary symptoms reduced from 58% to 29% [15].
Summary
Simple hydrodynamic physics can be used to help the clinician understand CSF physiology and valve mechanics. Despite advances in neurodiagnostic techniques the diagnosis and management of patients with probable INPH requires sound clinical judgement. In clinical practice we use clinical, radiological, ICP and CSF infusion tests to help select patients for shunt insertion. A standardised easily applied mini-battery assessment tool would be useful to provide an objective measure of treatment effect.
References
- Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with “normal” cerebrospinal fluid pressure: a treatable syndrome. N Engl J Med 1965;273:117-26. https://doi.org/10.1056/NEJM196507152730301
- Black P McL. Idiopathic normal pressure hydrocephalus: results of shunting in 62 patients. J Neurosurg 1980;52:371-7. https://doi.org/10.3171/jns.1980.52.3.0371
- Relkin N, Marmarou A, Klinge P, Bersneider M, Black P McL. Diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery 2005;57:S2, 4-16. https://doi.org/10.1227/01.NEU.0000168185.29659.C5
- Bradley WG Jr, Whittemore AR, Watanabe AS, Davis SJ, Teresi LM, Homyak M. Association of deep white matter infarction with chronic communicating hydrocephalus: implications regarding the possible origin of normal pressure hydrocephalus. Am J Neuroradiol 1991;12:31-9.
- Tullberg M, Jenson C, Ekholm S, Wikkelso C. Normal pressure hydrocephalus: vascular white matter changes on MR images must not exclude patients from shunt surgery. Am J Neuroradiol 2001;22:1665-73.
- Bradley WG Jr, Scalzo D, Queralt J, Nitz WN, Atkinson DJ, Wong P. Normal pressure hydrocephalus evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology 1996;198:523-9.
https://doi.org/10.1148/radiology.198.2.8596861 - Klinge PM, Berding G, Brinker T, Knapp WH, Samii M. A positron emission tomography study of Cerebrovascular reserve before and after shunt surgery in patients with idiopathic chronic hydrocephalus. J Neurosurg 1999;91:605-9. https://doi.org/10.3171/jns.1999.91.4.0605
- Eklund A, Agren-Wilson A, Andersson N, Bergenheim T, Koskinen L-OD, Malm J. Two computerized methods to analyze incracranial pressure B waves: comparison with traditional visual interpretation. J Neurosurg 2001;94:392-6. https://doi.org/10.3171/jns.2001.94.3.0392
- Stephensen H, Andersson N, Eklund A, Malm J, Tisell M, Wikkelso C. Objective B wave analysis in 55 patients with non-communicating and communicating hydrocephalus. J Neurol Neurosurg Psych 2005;76:965-70. https://doi.org/10.1136/jnnp.2004.039834
- Marmarou A, Bergsneider M, Klinge P, Relkin N, Black P McL. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57:S2,17-28. https://doi.org/10.1227/01.NEU.0000168184.01002.60
- Czosnyka M, Czosnyka ZH, Whitfield PC, Donovan T, Pickard JD. Age dependence of cerebrospinal pressure volume compensation in patients with hydrocephalus. J Neurosurg 2001;94:482-6. https://doi.org/10.3171/jns.2001.94.3.0482
- Boon AJ, Tans JT, Delwel EJ, Egeler-Peerdeman SM, Hanlo PW, Wurzer HA, Avezaat CJ, de Jong DA, Gooskens RH, Hermans J. Dutch normal pressure hydrocephalus study: prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. J Neurosurg 1997:87;687-93. https://doi.org/10.3171/jns.1997.87.5.0687
- Czosnyka Z, Czosnyka M, Richards HK, Pickard JD. Laboratory testing of hydrocephalus shunts – conclusion of the UK shunt evaluation programme. Acta Neurochir 2002;144:525-38.
- Zemack G, Romner B. Adjustable valves in normal-pressure hydrocephalus: a retrospective study of 218 patients. Neurosurgery 2002;51:1392-1402. https://doi.org/10.1097/00006123-200212000-00009
- Savolainen S, Hurskainen H, Paljarvi L, Alafuzoff I, Vapalahti MP. Five year outcome of normal pressure hydrocephalus with or without a shunt: predictive value of the clinical signs, neuropsychological evaluation and infusion test. Acta Neurochir 2002;144:515-23. https://doi.org/10.1007/s00701-002-0936-3
Box 1
Diagnostic testing
From this data several parameters can be calculated:
Sensitivity = a/(a+c) = 33/ 72 = 46%
Specificity = d/(b+d) = 20/23 = 87%
Likelihood ratio for positive result = sensitivity/ (1-specificity) = 46/(1-0.87) = 3.5
Positive predictive value = a/(a+b) = 33/36 = 92%
Negative predictive value = d/(c+d) = 20/59 = 34%
Prevalence of SR hydrocephalus = (a+c)/(a+b+c+d) = 72/95 = 76%
Pre-test odds of having SR hydrocephalus = Prevalence/(1-prevalence) = 0.76/(1 – 0.76) = 3.2
Post test odds of having SR hydrocephalus = Pre-test odds x Likelihood ratio = 3.2 x 3.5 = 11.2
Post-test probability of having SR hydrocephalus = Post test odds/ (Post test odds + 1) = 11.2/12.2 = 92%
If the Rcsf test had a very high sensitivity a negative result effectively rules out the diagnosis of shunt responsive (SR) hydrocephalus. This is not the case. The Rcsf test does have a high specificity. A positive result therefore makes the diagnosis of SR hydrocephalus very likely. Tests that produce big changes from the pre-test to post-test probabilities are likely to be clinically useful. The likelihood ratio of 3.5 is in the mid-range, but given that most patients investigated turned out to have SR hydrocephalus (high prevalence) the probability of having the target disorder was high if the Rcsf exceeded 18mmHg/ml/min. If a second independent test is performed with a different likelihood ratio, eg. B wave analysis of ICP recordings at 1mmHg threshold9 (Likelihood ratio = Sens/(1-Spec) = 0.78/(1 – 0.6) = 1.95), this can be used to adjust the post-test probability of having SR hydrocephalus:
Post test odds of having SR hydrocephalus = 3.2 x 3.5 x 1.95 = 21.8
Post test probability of having SR hydrocephalus = 21.8/22.8 = 96%
Therefore a combination of investigations can prove useful at helping to determine whether or not shunt insertion likely to be beneficial. Using Boon’s data at the 12mmHg/ml/min threshold, the likelihood ratio reduced to 1.4 and the post-test probability to 82%. Whilst this seems quite high it is only just better than clinical acumen alone (76% of patients with clinical features had SR hydrocephalus in this series).


