Introduction
The spectrum of parkinsonian syndromes is wide and, due to the lack of specific biomarkers, their diagnosis remains largely clinical. Disorders that are most commonly referred to as ‘atypical parkinsonism’ comprise progressive supranuclear palsy (PSP), multiple system atrophy (MSA), corticobasal degeneration (CBD) and dementia with Lewy bodies (DLB). The characteristic features of these disorders are well recognised. However, these features may be absent or ambiguous at initial diagnosis and the clinician may be uncertain about the L-dopa response. Many atypical parkinsonism cases initially resemble idiopathic Parkinson’s disease (PD).
Furthermore, clinicopathological studies have suggested varying and overlapping phenotypes between these disorders. Early diagnostic accuracy is not only important for patients and their families, particularly with respect to prognosis, but also imperative for researchers entering patients into clinical studies. Identification of patients in the pre-symptomatic phase is also essential for evaluation of potential disease modifying agents. There has been much discussion regarding abnormal imaging findings in atypical parkinsonism, but the question arises as to how useful these are in clinical practice . In this brief article, we will outline how structural and functional imaging can aid the diagnosis of atypical parkinsonism.
Part 1: Atypical parkinsonism
PSP
Pathologically PSP is characterised by neuronal loss, gliosis and the presence of microtubule-associated tau inclusions within neuronal and glial cells. A genome-wide association study in PSP has recently identified new loci potentially related to underlying disease pathogenesis.1 There are two main clinical subtypes: Richardson syndrome (PSP-R) and PSP-parkinsonism (PSP-P), which appear to differ pathologically as well as clinically. PSP-R is the classic phenotype: patients present with falls, executive dysfunction, eye movement abnormalities (initially with slowed saccades and evolving into supranuclear gaze palsy), dysarthria and marked postural instability. In PSP-P there is bradykinesia, limb and axial rigidity, and sometimes a jerky tremor at presentation. Signs may be asymmetric and initially be L-dopa responsive. Rarer subtypes include PSP-pure akinesia with gait freezing, PSP-corticobasal syndrome and PSP-frontotemporal dementia. The Movement Disorder Society Task Force is currently reviewing the diagnostic criteria for PSP and these should be published in 2015.
MSA
There are two main clinical subtypes of MSA: MSA with parkinsonism (MSA-P) and MSA with cerebellar signs (MSA-C), with progressive autonomic failure and falls being common to both. MSA-P may be difficult to differentiate clinically from PD although the former’s signs are usually more symmetrical. MSA-C patients present with gait ataxia, dysarthria, cerebellar and oculomotor dysfunction, often with little evidence of parkinsonism. Other features may include stridor and pyramidal signs. MSA is generally poorly L-dopa responsive and a minority of patients have cognitive dysfunction.2
In common with PD and DLB, pathologically MSA is an alpha-synucleinopathy, except that oligodendroglia rather than neurones are affected. Although these lesions are widespread they are predominantly located within olivopontocerebellar regions in MSA-C and within striatonigral regions in MSA-P.3 Furthermore, the burden of these inclusions is greater with increasing disease duration and severity. Familial MSA is very rare, but variants in the alpha-synuclein gene have been associated with an increased disease risk.4
CBD
CBD is possibly the most challenging atypical parkinsonism to diagnose accurately. The classic presentation is with a corticobasal syndrome (CBS) consisting of asymmetric akinetic-rigid parkinsonism, ideomotor apraxia, dystonia, myoclonus and the alien limb phenomenon. However, post-mortem studies have shown that only 50% of CBS patients clinically diagnosed during life pathologically had CBD, with other causes of CBS including PSP, Alzheimer’s disease and frontotemporal dementia (FTD). Furthermore, rarer CBD phenotypes include FTD, progressive non-fluent aphasia and Richardson syndrome. The clinical diagnostic criteria for CBD were updated in 2013 and cases can be divided into probable and possible.5 Pathologically there are tau-positive inclusions with cortical and striatal neuronal and glial cells with marked neuronal cell loss in frontoparietal and nigral regions.
DLB
Like PD and MSA, DLB is an alpha-synucleinopathy. Parkinson’s disease dementia and DLB are pathologically considered as a continuum with little change in the distribution or quantity of Lewy bodies and neurites. Clinically DLB is differentiated by the predominance of dementia at presentation or within 12 months of motor symptoms.6 In DLB the cognitive impairment is characterised by deficits in attention and executive function. Other features include fluctuations of cognition, visual hallucinations and sensitivity to neuroleptic and anticholinergic medication.
Part 2: Structural imaging
In the past, patients with dopa-unresponsive parkinsonism were investigated with conventional structural brain imaging to identify cases of cerebrovascular disease and also exclude rare causes of parkinsonism such as hydrocephalus. More recently MRI has become a helpful adjunct in the differentiation of the atypical parkinsonian syndromes. While the specificity of the abnormal signs described may be high their sensitivity in early disease, particularly using a 1.5 Tesla scanner, is less impressive. Nevertheless, they can all serve as pointers towards the diagnosis in uncertain cases.
PSP
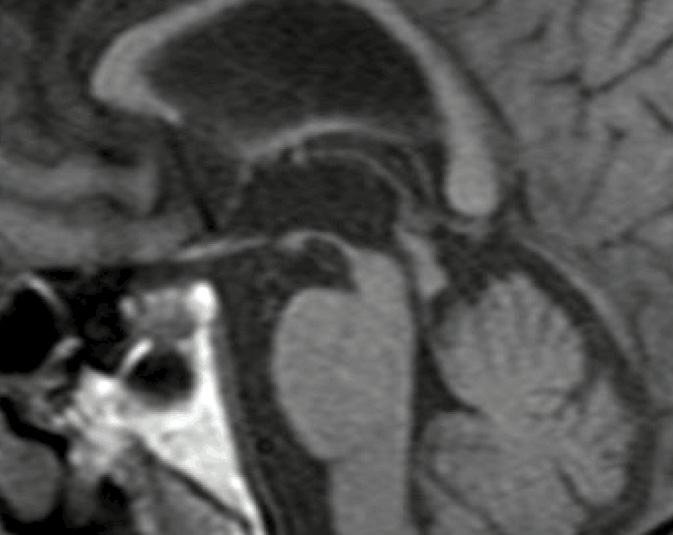
Atrophy of the mid-brain, superior cerebellar peduncle, frontal and parietal lobes, and dilatation of the 3rd ventricle have all been reported in PSP. Signs of midbrain atrophy have been variously described as the ‘morning glory flower sign’ (concavity of the lateral midbrain tegmentum on axial scans), or the ‘hummingbird’ or ‘penguin-silhouette sign’ (where the shape of the midbrain tegmentum represents the bird’s head and the pons represents its body on mid-sagittal sequences). Various advanced MRI techniques such as magnetic resonance volumetry (MRV) and voxel-based morphometry (VBM), diffusion-tensor imaging (DTI), magnetic resonance spectroscopy (MRS) have been used to study PSP in a research setting. However, a recent study of pathologically confirmed PSP, MSA and PD cases reported that a simple mid-sagittal midbrain measurement of <9.35mm and a ratio of midbrain to pons of <0.52 on conventional MRI had 100% specificity for PSP.7
MSA
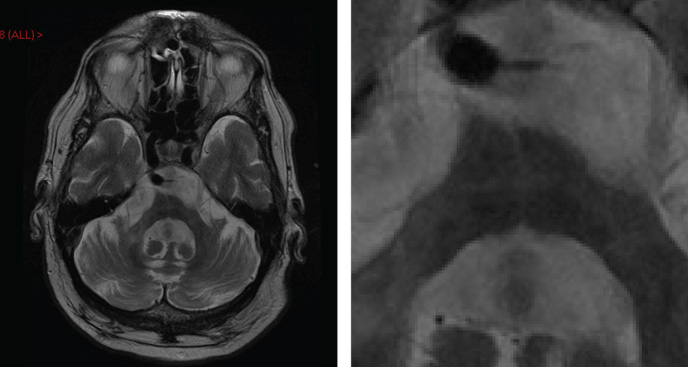
In MSA atrophy is most evident in patients with well-established disease. Structural imaging changes recognised in MSA-P include high T2-weighted signal within the posterolateral putamen, reflecting gliosis and iron deposition.8 In MSA-C the atrophy is infratentorial affecting the pons, middle cerebellar peduncle and cerebellum. Axial T2-weighted images can reveal a cross in the pons, reflecting disruption of transverse pontocerebellar tracts, and is known as the ‘hot cross bun’ sign. Whilst this is usually associated with MSA-C, it has also been recognised in other neurodegenerative disorders (including spinocerebellar ataxia types 2 and 3).
CBD and DLB
Asymmetric frontoparietal atrophy can be seen with CBD. Usually the lack of midbrain atrophy helps to differentiate this from similar patterns of atrophy seen in PSP. There may be generalised cortical atrophy in DLB but the most useful finding is often preservation of medial temporal structures, which helps in differentiation from Alzheimer’s disease.
Part 3: Functional imaging
Over the past 15 years functional imaging has established a role in the differentiation of PD from non-degenerative disorders.9 Non-degenerative disorders clinically mistaken for PD include essential tremor, dystonic tremor, and drug-induced, vascular or psychogenic parkinsonism.
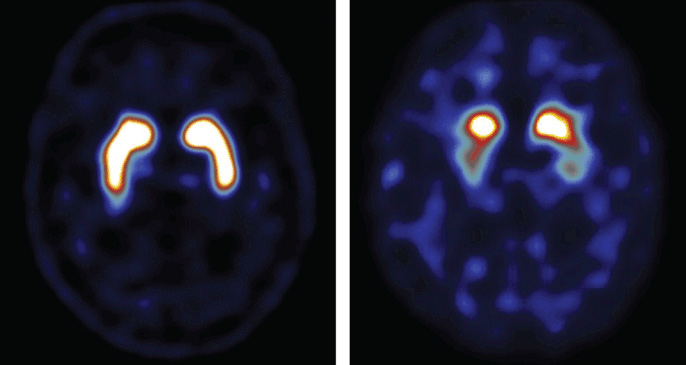
18F dopa PET indirectly measures nigrostriatal function and is abnormal in degenerative parkinsonism. However, it is dopamine transporter (DaT) SPECT scanning that has become widely available and utilised in Europe. DaT is a sodium chloride-dependent transmembrane protein located on the presynaptic cell surface. The most commonly used DaT-binding radioligand is FPCIT.
By the time patients present with motor features of PD they have lost up to 80% of their dopaminergic neurones. Consequently DaT SPECT is abnormal at diagnosis, and in the pre-motor phase. Furthermore, visual assessment techniques are equally reliable as semi-quantitative measures in FPCIT SPECT making the interpretation technically easier.
DaT SPECT is also abnormal in atypical parkinsonism, notably PSP, MSA and DLB. It has been suggested that patterns of abnormal radioligand uptake differ between types of degenerative parkinsonism. For example, in PD there is reduced uptake in the putamen before the caudate is affected, whereas in PSP there can be uniform striatal involvement at presentation. However, it remains impossible to reliably differentiate between PD, PSP and MSA using DaT SPECT alone.10
DaT SPECT is abnormal in DLB but does not distinguish it from PD dementia. It is slightly less useful in CBD with 10% of patients having normal scans.11
Imaging of the post-synaptic D2 receptors with IBZM SPECT has also been studied in combination with DaT SPECT in atypical parkinsonism. IBZM SPECT is often abnormal in MSA and PSP, but tends to be normal in CBD and DLB. If abnormal, it carries a high positive predicative value but it does not differentiate between MSA and PSP and has been used less frequently in recent years.
Part 4: Other techniques
In PD transcranial sonography (TS) reveals hyperechogenicity of the substantia nigra and has a positive predictive value of 93%.12 Its clear advantage is that it is non-invasive and cheap, but it requires an adequate temporal bone window. Furthermore, 10% of the population have abnormal susbstantia nigra echogenicity. In MSA a combination of striatal hyperechogenicity and normal echogenicity of the substantia nigra can distinguish MSA-P from PD.13 TS is also abnormal in PSP and CBD.
Another imaging method that has been studied in atypical parkinsonism is MIBG myocardial scintigraphy. This is usually reduced in PD and normal or slightly low in MSA-C.14 However, this technique has largely remained a research tool.
Conclusions
The importance of early and accurate diagnosis in neurodegenerative disorders cannot be overstated. The spectrum of atypical parkinsonian disorder is wide and until such time as reliable biomarkers are identified these disorders will continue to be diagnosed on clinical grounds. The challenge is greatest in early disease when characteristic clinical features may be subtle, if present at all. The recognised changes on structural imaging may not be present in these early stages but can certainly inform the diagnostic process in individual cases. Functional dopaminergic imaging also has a role, and has the advantage of being abnormal at an earlier clinical stage, but does not allow reliable differentiation between disorders.
References
- Höglinger GU, Melhem NM, Dickson DW, Sleiman PMA, Wang L-S, Klei L, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011 Jul;43(7):699–705.
- O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. Oxford University Press; 2008 May;131(Pt 5):136–72.
- McCann H, Stevens CH, Cartwright H, Halliday GM. Parkinsonism and Related Disorders. Elsevier Ltd; 2014 Jul 20;20(S1):S62–7.
- Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, Revesz T, et al. The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol. Blackwell Publishing Ltd; 2012 Feb;38(1):4–24.
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. Lippincott Williams & Wilkins; 2013 Jan 29;80(5):496–503.
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. Lippincott Williams & Wilkins; 2005. pp. 1863–72.
- Massey LA, Jäger HR, Paviour DC, O’Sullivan SS, Ling H, Williams DR, et al. The midbrain to pons ratio: a simple and specific MRI sign of progressive supranuclear palsy. Neurology. Lippincott Williams & Wilkins; 2013 May 14;80(20):1856–61.
- Schrag A, Good CD, Miszkiel K, Morris HR, Mathias CJ, Lees AJ, et al. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology. 2000 Feb 8;54(3):697–702.
- Benamer TS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord. 2000 May;15(3):503–10.
- Booth TC, Nathan M, Waldman AD, Quigley AM, Schapira AH, Buscombe J. The Role of Functional Dopamine-Transporter SPECT Imaging in Parkinsonian Syndromes, Part 2. AJNR Am J Neuroradiol. American Society of Neuroradiology; 2014 Jun 12.
- Cilia R, Rossi C, Frosini D, Volterrani D, Siri C, Pagni C, et al. Dopamine Transporter SPECT Imaging in Corticobasal Syndrome. Dawson TM, editor. PLoS ONE. Public Library of Science; 2011;6(5):e18301.
- Gaenslen A, Unmuth B, Godau J, Liepelt I, Di Santo A, Schweitzer KJ, et al. The specificity and sensitivity of transcranial ultrasound in the differential diagnosis of Parkinson’s disease: a prospective blinded study. Lancet Neurol. 2008 May;7(5):417–24.
- Berg D, Godau J, Walter U. Transcranial sonography in movement disorders. Lancet Neurol. 2008 Nov;7(11):1044–55.
- Chung EJ, Lee WY, Yoon WT, Kim BJ, Lee GH. MIBG scintigraphy for differentiating Parkinson’s disease with autonomic dysfunction from Parkinsonism-predominant multiple system atrophy. Mov Disord. Wiley Subscription Services, Inc., A Wiley Company; 2009 Aug 15;24(11):1650–5.

