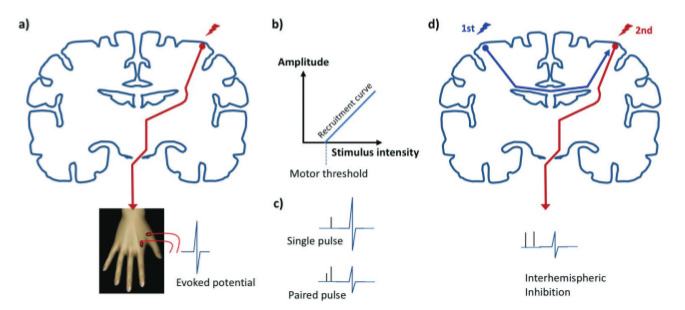
Transcranial Magnetic Stimulation (TMS) is a non-invasive technique whereby an electro-magnetic coil held over the scalp is used to induce a brief electrical current in the cortex of the underlying brain. TMS may be used in a number of ways to gain in vivo insights into brain physiology and has provided a window into the cascade of physiological changes that occur following stroke. When stimulating the primary motor cortex in a healthy subject stimuli of an intensity above the motor threshold will give rise to a detectable evoked potential in a peripheral muscle. As the stimulus intensity is gradually increased the evoked potentials increase in amplitude up to a maximum, giving rise to a measurable recruitment curve. The motor threshold and the recruitment curve both provide measures of the excitability of the corticospinal tract. This incorporates the excitability of axons within the motor cortex, synaptic inputs onto pyramidal cells and the spinal alpha motor neuron pool. In paired pulse TMS a sub-threshold pulse is delivered a few milliseconds before a second suprathreshold pulse, both through the same coil. The first pulse conditions the response to the second, resulting in inhibition or facilitation depending upon the inter-stimulus interval and reflecting the activity in intracortical regulatory circuits. If two separate TMS coils are used then one may use a similar test-conditioning approach to explore inter-regional interactions, such as interhemispheric inhibition between the two primary motor cortices (Figure 1). Alternatively pulses may be delivered during a motor task, and the resulting effect on behaviour used to infer the stimulated region’s role in task performance, for example during a simple reaction time or movement selection task. See Reis et al1 for a summary of these techniques and their physiological basis.
Strokes that cause hemiparesis usually disrupt the corticospinal tract, and when testing the stroke hemisphere this may manifest as increased motor thresholds, flattened recruitment curves, or alternatively as the inability to elicit an evoked potential at all. An absent evoked potential in the acute post-stroke period is a poor prognostic indicator for motor recovery2,3 and at that stage these markers of corticospinal excitability, when obtainable, correlate strongly with clinical measures of motor performance.4 If these corticospinal excitability measures improve then they usually do so over the early weeks post-stroke, presumably reflecting spontaneous biological recovery around the infarct, and the physiological change is reflected in clinical improvement in this group. It has been a fairly consistent finding that the correlation between corticospinal excitability and clinical status declines with time, which may reflect reduced reliance on the original corticospinal projection once the network generating the motor output has reorganised. Some studies have shown hyper-excitability of the corticospinal tract from the non-stroke hemisphere during the acute phase in more severely affected patients, which is interpreted by some as a form of diaschisis.
Paired pulse measures have fairly reliably shown reduced inhibitory activity in the intracortical circuits after stroke. Such apparent disinhibition is hard to interpret in the stroke hemisphere, as these measures depend upon an unconditioned evoked potential of reasonable amplitude which may not be available or may require high stimulus intensities. However no such technical issue affects the intact hemisphere, and the absence or reduction of inhibition when tested in the contralesional primary motor cortex suggests a reduction in the tonic level of GABA-ergic inhibitory activity in intracortical circuits that extends far beyond the site of the stroke. In one study such intracortical disinhibition of the non-stroke hemisphere was seen in patients with cortical but not subcortical infarction,5 suggesting that this phenomenon may relate to interruption of the transcallosal projection between the motor cortices. Few longitudinal studies of intra-cortical excitability have been performed but on the basis of the data available it seems that in the acute period disinhibition is seen regardless of clinical status, but that by three months it has resolved in those patients with a better clinical outcome, such that a clinical–physiological correlation emerges at around that time.6 A recent meta-analysis of TMS studies has shown no overall abnormality of excitability in the intact hemisphere:7 however if disinhibition were seen only in more severely affected patients then one may see clinical correlation without a group effect.
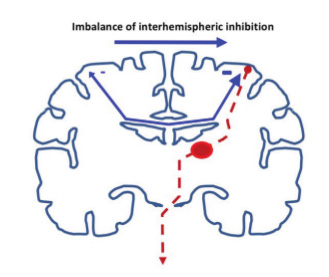
It is recognised that in healthy humans there is tonic inhibition of each hemisphere by its opposite, a situation that is likely to be important in the generation of unimanual versus bimanual movements. When this inter-hemispheric interaction is measured at rest with two TMS coils using a paired pulse conditioning approach there is robust inhibition. When tested in healthy subjects during a reaction time paradigm the baseline inhibition disappears or reverses as the onset of movement approaches. Murase and colleagues8 found that such switching-off of interhemispheric inhibition was impaired in stroke patients, and that the extent of residual interhemispheric inhibition was greater in more severely affected patients. This result has been interpreted as suggesting that after stroke there is an imbalance of such interhemispheric interactions, with pathological inhibition of the recovering stroke hemisphere by the non-stroke hemisphere.
Interpretation of the physiological changes observed after stroke remains a matter of debate. TMS as a technique operates at the level of the whole system, drawing inferences from the effect of manipulations on the overall corticospinal output, but the pathological changes observed may be the result of dysfunction at one or more of several levels. These may include the effects of cytotoxic changes on local neurochemistry, altered inhibitory vs excitatory synaptic activity, or diaschisis due to disruption of inter-regional tracts. The TMS finding of wide- spread intracortical disinhibition is in keeping with MR Spectroscopy studies that show reduced cortical GABA content during this period after stroke.9 A rapid reduction in GABA is also seen in healthy humans as a response to motor training or to experimental deafferentation of one arm by ischaemic nerve block, which likewise causes intracortical disinhibition as assessed by TMS. In those contexts it is felt that such disinhibition creates a more favourable environment for synaptic plasticity to occur, and it is tempting to conclude that the same is occurring in the post-stroke period as a way of driving reorganisation of the motor output. It is also conceivable that disinhibition allows cortical regions that are disconnected from their usual corticospinal output projection to access instead the horizontal cortico-cortical connections that are prevalent in cortical layers 2 and 3, thereby reaching an alternative output projection. Such a phenomenon would allow for the shifts in cortical motor output maps that are well documented after stroke.10 In one longitudinal study the correlation between disinhibition and clinical status was strong at three months but then became weaker in the chronic phase.6 We interpreted this as suggesting that ongoing disinhibition becomes less important for motor function as the reorganised motor network becomes better established with time. Direct evidence for such a process is lacking however, and some would argue that disinhibition is an epiphenomenon rather than an adaptive response to injury. This alternative conclusion would be supported by the observation that reduced intracortical inhibition as measured by paired pulse TMS may be observed in other pathological states, including dystonia and Attention Deficit Hyperactivity Disorder.
The concept of an imbalance between the two hemispheres and of excessive interhemispheric inhibition of the stroke hemisphere has gained a lot of traction and has provided the rationale for therapeutic approaches that aim to redress it. Non-invasive brain stimulation, either by repetitive TMS or transcranial direct current stimulation (tDCS), can induce measurable changes in cortical excitability that outlast the period of stimulation. Depending on the stimulation paradigm used one can induce either increases or reductions lasting minutes or in some cases hours. The most common approaches are either to apply excitatory stimulation to the stroke hemisphere or alternatively inhibitory stimulation to the non-stroke hemisphere (Figure 2), the aim being to enhance the response to conventional therapy by stimulating either before or during treatment. The concept of an overactive non-stroke hemisphere resonates with the widely reproduced functional imaging finding of increased movement-related brain activation on that side in more severely affected patients, which may normalise as clinical recovery progresses.6
It is by no means clear however that this contralesional activity is the same phenomenon as that which generates pathological inhibition of the recovering hemisphere, or that it is necessarily maladaptive. There is evidence that at least some regions on the non-stroke side may support movement of the paretic side, such as the contralesional dorsal premotor cortex which displays increased functional connectivity to the primary motor cortex of the stroke in more affected patients and appears to support hand movement.11,12 Furthermore a recent study suggested that reducing intact hemisphere excitability may in fact be detrimental to upper limb function in more impaired patients.3
As the role of contralesional brain regions in movement appears to differ depending upon factors such as clinical severity, extent of corticospinal tract disruption and possibly stroke location it would seem that reducing excitability on that side equally in all stroke patients may represent rather a blunt therapeutic approach. This is especially true of tDCS, whose effects incorporate most of the stimulated hemisphere. However, positive studies may be found in the literature for both repetitive TMS and tDCS when applied to either side of the brain (sometimes both), and although a comprehensive review of the outcomes is beyond the scope of this article the most promising approach appears to be inhibition of the non-stroke hemisphere by tDCS.13 Non-invasive brain stimulation has not as yet entered routine clinical practice however, and there are a number of reasons why this may be, such as the large number of stimulation protocols available and uncertainty regarding the optimal time to apply stimulation. However, for the reasons discussed above it is important to consider the heterogeneity of the clinical syndrome when designing further trials. Opinions differ as to whether progress will be made using a ‘one size fits all’ design, the hope being that larger sample sizes will take care of heterogeneity, or alternatively whether targeting stimulation according to clinical and physiological factors would have a greater chance of success. This is likely to be worth getting right, as a large negative study would present an obstacle to further investigation in this field. It is likely to be the case that applying brain stimulation to the right patients could significantly enhance the outcome of post-stroke rehabilitation, with a clinically meaningful reduction in resulting impairment and disability.
References
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 2008; 586(2):325-51.
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional Potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007;130:170-180.
- Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex. 2012 Nov;22(11):2662-71.
- Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the ‘affected’ and ‘unaffected’ hemispheres in human stroke. Brain Research 1998; 803:1-8.
- Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol 2002; 113:936-943.
- Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of Motor Output Reorganization after Hemispheric Stroke Suggested by Longitudinal Studies of Cortical Physiology. Cereb Cortex 2008; 18:1909- 1922.
- McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimul. 2017 Jul – Aug;10(4):721-734.
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 2004; 55:400-409.
- Blicher JU, Near J, Næss-Schmidt E, Stagg CJ, Johansen-Berg H, Nielsen JF, Østergaard L, Ho YC. GABA levels are decreased after stroke and GABA changes during rehabilitation correlate with motor improvement. Neurorehabil Neural Repair 2015 Mar-Apr;29(3):278-86.
- Delvaux V, Alagona G, Gerard P, De Pasqua V, Pennisi G, de Noordhout AM. Post-stroke reorganization of hand motor area: a 1-year prospective follow-up with focal transcranial magnetic stimulation. Clin Neurophysiol 2003; 114:1217-1225
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Teo J, Weiskopf N, Driver J, Rothwell JC, Ward NS. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci 2010; 30(36):11926-37.
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A 2002; 99:14518- 14523.
- Kang N, Summers JJ, Cauraugh JH. Transcranial direct current stimulation facilitates motor learning post- stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016 Apr;87(4):345-55.
