Intracranial dural arteriovenous fistulae (DAVF) are uncommon lesions. Their true incidence is unknown, although selected series suggest that they occur only one tenth as frequently as intraparenchymal arteriovenous malformations (AVM) [1]. Since many may remain clinically silent or involute spontaneously the incidence may be an underestimate [2]. DAVF tend to present later in life than AVMs, lending support to the theory that these are acquired lesions, although presentation can be at any age [3]. A presentation with aggressive neurological symptoms is more common in males.
The fistula represents an abnormal connection between dural arteries or pachymeningeal branches of cerebral arteries and dural veins. Occasionally, as a fistula grows or becomes more diffuse, pial recruitment from parenchymal vessels can occur. In addition, dilatation of cortical veins may occur, predisposing the patient to intracranial haemorrhage. Previous surgery, ear infection and head trauma have all been cited as potential causes, although the common predisposing factor appears to be venous sinus thrombosis [4]. Venous thrombosis promotes venous hypertension, which acts as the initiating factor opening up microscopic vascular connections within the dura [5]. Maturation of these channels secondary to progressive venous stenosis or occlusion results in the development of direct shunts between the arteries and dural veins [6]. In addition, a second complementary mechanism of DAVF evolution may occur with the release of angiogenic growth factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) promoting neovascularisation and development of a DAVF.
Presentation and natural history
A wide spectrum of symptoms exists, ranging from the benign to the more aggressive. Individual lesions may regress spontaneously or follow a benign course over years. Drainage of a petrous region DAVF to the transverse or sigmoid sinus commonly produces pulsatile tinnitus, sometimes in association with an audible bruit. Depending upon the pattern of venous drainage such patients may be managed conservatively. Cavernous sinus DAVFs may develop orbital signs such as congestion, chemosis and ophthalmoplegia. Treatment is usually undertaken to protect against ocular and visual complications.
More aggressive behaviour may manifest as focal neurological deficits, a dementia-type of syndrome or cerebral haemorrhage, including subarachnoid, subdural or intraparenchymal bleeds [7]. Such features are usually considered to be due to venous hypertension, although neurological deficits may be secondary to arterial steal [8]. In a meta-analysis of 360 dural AVFs the tentorial incisura was the most ominous location, with 31 out of 32 cases associated with haemorrhagic or non-haemorrhagic stroke [7]. However the pattern of venous drainage was considered of paramount importance in predicting aggressive behaviour [7]. Angiographic features that appear to be associated with aggressive behaviour comprise leptomeningeal retrograde venous drainage, variceal or aneurysmal venous structures, and galenic venous drainage [7,9]. Treatment of DAVFs with these features warrants serious consideration.
In a longitudinal study of 117 patients with no evidence of cortical venous drainage followed over 348 patient years, observational management rather than intervention was chosen for 73 (62.4%). Five of these patients were lost to follow up. 50 patients underwent repeat angiography due to a change in symptoms. In two of these cases cortical venous reflux was seen. This appears to indicate that the risk of conversion from a benign to an aggressive DAVF is small but sufficient to warrant repeat angiography if the clinical picture appears to progress [9].
PET based regional cerebral blood flow (rCBF) studies indicate that impaired rCBF may be important in the pathogenesis of neurological symptoms. In patients with normal cortical venous drainage, values for regional cerebral blood flow (rCBF), regional cerebral metabolic rate of oxygen (rCMRO) and regional oxygen extraction fraction (rOEF) were normal. Studies in patients with neurological symptoms and cortical venous drainage, showed reduced rCBF and mildly or markedly increased rOEF [10].
Classification
Various classification methods have been adopted that attempt to explain the significance of the angiographic anatomy; namely, the pattern of venous drainage and the clinical presentation and outcome. The two commonly used classification systems are shown in Tables 1 and 2. With only three sub-types the Borden classification is user friendly [3]. However, the Cognard system is more detailed and elaborates on the direction of flow, whether normal (anterograde) or retrograde and the presence or absence of cortical venous recruitment. Such definition enables more accurate comparison of clinical and radiological parameters. In addition, spinal perimedullary venous drainage is specifically recognised [11].
In a retrospective review of 102 DAVFs in 98 patients Davies et al. reported a significant correlation between Borden type and clinical presentation [12]. The progression of disease severity with lesion type was tracked in Cognard`s cohort of 205 patients. 27 patients had a type IIa fistula. Of these 10 (37%) had aggressive clinical symptoms manifested as headache, papilloedema or visual disturbance. In the 10 patients with type IIb DAVFs, 3 (30%) followed an aggressive course. 12 of 18 (67%) patients with type II a+b disease showed aggressive symptoms, including an intracranial haemorrhage in 1 case. 19 (76%) of 25 patients with type III disease had aggressive symptoms, including 10 (40%) presenting with haemorrhage. Twenty nine patients had type IV DAVFs with direct venous drainage into a cortical vein with ectasia. Of these, 28 (97%) had aggressive symptoms, of which 19 (66%) had haemorrhage. 6 of these patients had symptoms attributable to mass effect from the ectatic vein. The presence of direct cortical venous drainage was therefore a strong predictor of haemorrhage. Of those 12 patients with spinal perimedullary venous drainage, 6 presented with myelopathy.
Imaging
CT, MRI and angiography all have roles to play in the investigation of patients with a possible DAVF. Because the clinical and imaging features can be non-specific, the diagnosis of a DAVF is often delayed or missed. Occasionally plain films can demonstrate grooving within the skull vault due to chronic compression from enlarged middle meningeal vessels. If haemorrhage is suspected, non-enhanced CT is a pre-requisite. Venous congestion may appear as an area of low density on CT. In most institutions CT is more readily available and cheaper than MRI and so becomes the first-line investigation of patients presenting with tinnitus, headache or other vague neurological symptoms. Multi-detector CT angiography (MDCTA) can now provide high resolution detail of vascular anatomy. In the investigation of tinnitus it has the additional advantage that it can detect inner and middle ear abnormalities such as aberrant vascular anatomy or glomus tumours. Linear bony defects formed by enlarged emissary veins, similar to the grooving abnormality seen on plain film, can indicate the presence of a fistula [13]. MDCTA, because of its rapid acquisition, has a temporal advantage over static CT. This is important because of the likelihood of altered flow dynamics within a fistula. Subtle changes in contrast intensity of the cerebral vessels may be evident [14]. Arterialised venous blood within the veins draining a DAVF has increased density when compared to non-arterialised blood. Careful scrutiny of the source images with narrow window settings are required to make this distinction.
T2 weighted MRI is more sensitive to the white matter changes of venous congestion or infarction when compared to CT. It has the drawback of being less sensitive to the changes of acute haemorrhage. If dilated cortical veins are present they may be seen on conventional spin echo sequences and visualised using MR angiographic techniques such as phase contrast venography or contrast enhanced MR angiography. Benign disease, without cortical venous reflux can be missed using both CT and MRI. Conventional catheter angiography therefore remains the investigation of choice if there is a strong clinical suspicion of a fistula.
Treatment
Treatment is dependent on the clinical picture and the grade of fistula. A multi-disciplinary approach involving a neurosurgeon and neuroradiologist is required. A DAVF without angiographic evidence of retrograde sinus or cortical venous drainage and presenting with a well-tolerated or non-disabling tinnitus can be managed conservatively. Techniques such as occipital artery or carotid manual compression have been reported to occasionally lead to obliteration of the DAVF although this may correspond to the natural history of the disease. If possible, patients should avoid anti-platelet agents which might prevent thrombosis. A change in symptoms warrants repeat assessment with formal angiography.
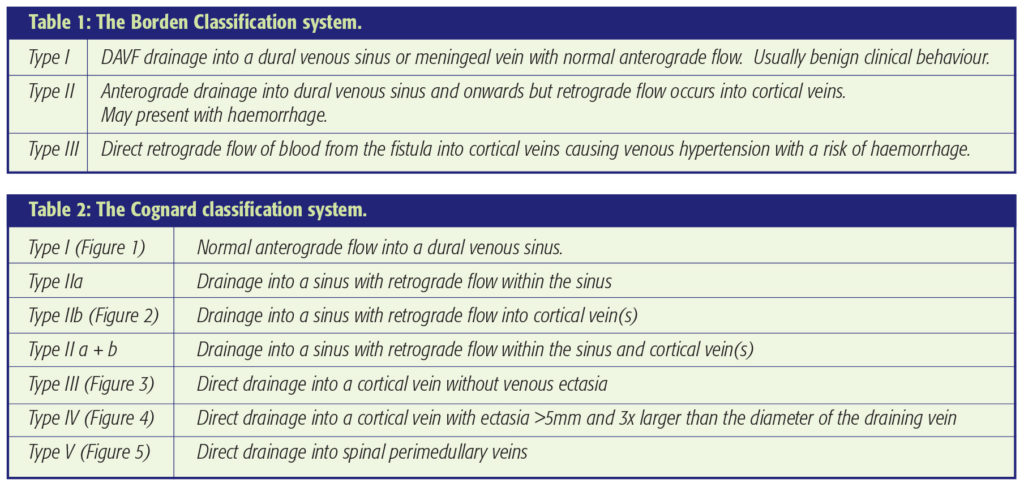
Both surgical and endovascular techniques have proven efficacy for more troublesome DAVFs. At the benign end of the spectrum, particulate trans-arterial embolisation may afford palliation (Figure 1). This can be regarded as fairly low risk but the risks should not exceed the natural history of the disease. In addition, further recruitment of fistulous channels may cause re-emergence of symptoms at a later date. For more severe disease and particularly for those patients presenting with intracranial haemorrhage or progressive neurological symptoms, treatment is indicated (Figures 2, 3 and 4). If the DAVF drains directly to cortical veins without involvement of the sinus, surgical disconnection of the arterialised draining vein(s) can be employed. This minimises the risk of future intracranial haemorrhage and is facilitated by the use of neuronavigation to localise the craniotomy. Surgery can be combined with pre-operative particle embolisation one to two days before surgery to reduce the risk of intra-operative bleeding and in some cases permit complete resection of the involved dura.
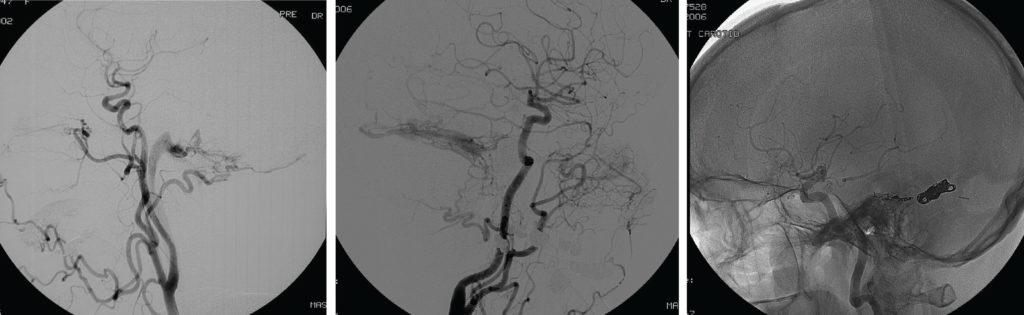
Figure 2a middle: Type IIb DAVF – A lateral projection left common carotid artery injection. This 68-year-old lady presented with progressive tinnitus and ataxia a few weeks after an ear infection. She had sustained a minor head injury 2 years previously. The angiogram shows a DAVF supplied by transmastoid branches of the occipital artery, the middle meningeal artery, the ascending pharyngeal artery and tentorial dural branches of the internal carotid artery. Venous drainage to the transverse sinus was ‘trapped’ due to distal sinus occlusion. Retrograde cortical venous drainage occurred. Transvenous occlusion of the sinus and associated fistula was planned. This required a small craniectomy and direct placement of the microcatheter in the transverse sinus.
Figure 2b (right) bottom: Coil occlusion of the transverse and petrosal sinuses obliterated the DAVF as shown on this 6 month follow-up angiogram.
Surgery becomes more problematic when a sinus is involved, and has largely been superseded by the use of transvenous and/or transarterial endovascular approaches [15]. The goal of treatment is to occlude the nidus of the fistula. This requires penetration of the venous side of the fistula. In cases where multiple dural feeders are present, a trans-arterial approach can promote the formation of collateral vessels and make subsequent treatment attempts more difficult. Where a transarterial approach is adopted liquid adhesive agents may be more effective at venous penetration from the arterial side to produce a cure. A retrograde approach into the venous side of a fistula requires careful negotiation of femoral catheters through the right atrium, and then deployment of coils into the receiving sinus or cortical vein. A thorough appreciation of the cerebral venous drainage anatomy is required before considering sacrifice of a vessel. When sacrificing a dural sinus it is important to ensure that any cortical venous reflux is abolished in order to minimise the risk of intracranial haemorrhage. This technique is commonly employed to treat fistulas of the cavernous sinus via a petrosal sinus. Occasionally, a sinus may no longer communicate with an internal jugular vein because of thrombosis. In these cases a direct percutaneous approach can be successful via a burr hole (Figure 2b) or orbital cut down procedure in the case of a carotico-cavernous fistula.
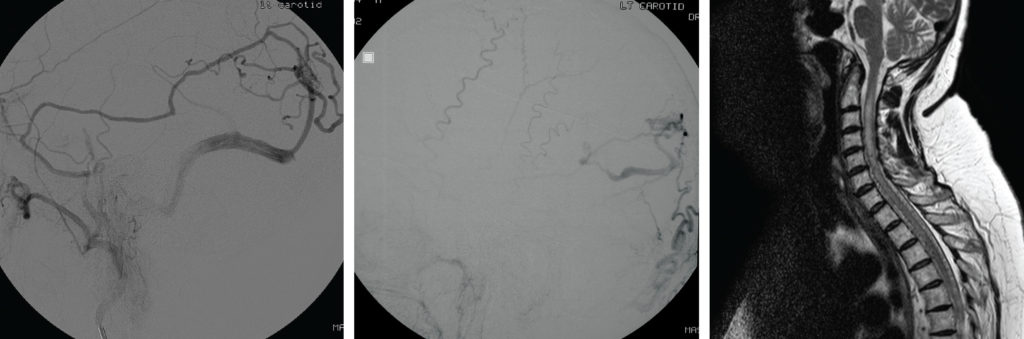
Figure 4 (middle): Type IV DAVF – A 74-year-old man presented with a coma producing intracerebral haemorrhage. An AP projection left external carotid artery injection DSA showed a DAVF draining into ectatic cortical veins.
Figure 5 (right): Type V DAVF – MRI scan – This 59-year-old lady presented with an 18 month progressive myelopathy leaving her wheelchair bound. A sagittal T2 weighted MRI demonstrated high signal within an expanded cervical cord. Numerous small flow voids lying both ventral and dorsal to the cord were noted. An angiogram revealed a DAVF with drainage to spinal peri-medullary veins. Partial occlusion with a dilute 20/80 NBCA/ Lipiodol injection led to some improvement in the myelopathic symptoms.
Radiosurgery has been used in the treatment of DAVFs. Söderman et al. treated 53 patients over a 25 year period with gamma knife radiosurgery [16]. 36 patients had aggressive shunts exhibiting cortical venous drainage. 19 of these presented with haemorrhage. 41 patients were followed up by formal angiography and 28 DAVFs were obliterated. The risk of haemorrhage exists however until complete obliteration has occurred. Radiosurgical treatment should therefore be considered if occlusion by surgical or endovascular means is not possible or carries unacceptable risks.
Summary
DAVFs can present in a variety of ways and their diagnosis can be missed on conventional cross-sectional imaging. Conven-tional catheter angiography remains the investigation of choice if the diagnosis is clinically suspected. A spectrum of pathology exists ranging from the benign to life-threatening. Treatment is indicated in more aggressive disease. This is characterised by cortical venous reflux on angiographic investigations. A multi-disciplinary app-roach is required before considering treatment, which can be surgical, endovascular or occasionally radiosurgical.
References
- Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology 1969;93:1071-8. https://doi.org/10.1148/93.5.1071
- Luciani A, Houdart E, Mounayer C, Saint Maurice JP, Merland JJ. Spontaneous closure of dural arteriovenous fistulas: report of three cases and review of the literature. AJNR 2001:22;992-6.
- Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 82;1995:166-79. https://doi.org/10.3171/jns.1995.82.2.0166
- Kwon BJ, Han MH, Kang HS, Chang KH. MR imaging features of intracranial dural arteriovenous fistulas: relations with venous drainage patterns. AJNR 2000;26:2500-7.
- Uranishi R, Nakase H, Sakaki T. Expression of angiogenetic growth factors in dural arteriovenous fistula. J Neurosurg 1999:91;781-6. https://doi.org/10.3171/jns.1999.91.5.0781
- van Dijk JMC, Willinsky RA. Venous congestive encephalopathy related to cranial dural arteriovenous fistulas. Neuroimaging Clin of North America 2003;13:55-72. https://doi.org/10.1016/S1052-5149(02)00063-1
- Awad IA, Little RJ, Akrawi WP, Ahl J.Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg 1990:72;839-50. https://doi.org/10.3171/jns.1990.72.6.0839
- Lasjaunias P, Chiu M, TerBrugge K, Tolia A, Hurth M, Bernstein M. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurgery 1986:64;724-30. https://doi.org/10.3171/jns.1986.64.5.0724
- Satomi J, van Dijk JMC, TerBrugge K, Willinsky RA, Wallace MC. Benign cranial dural arteriovenous fistulas: outcome of conservative management based on the natural history of the lesion. J Neurosurg 2002:97;767-70. https://doi.org/10.3171/jns.2002.97.4.0767
- Iwama T, Hashimoto N, Takagi Y, Tanaka M, Yamamoto S, Nishi S, Hayashida K. Haemodynamic and metabolic disturbances in patients with intracranial dural arteriovenous fistulas: positron emission tomography evaluation before and after treatment. J Neurosurg 1997:86:806-11. https://doi.org/10.3171/jns.1997.86.5.0806
- Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, Chiras J, Merland JJ. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995:194;671-80. https://doi.org/10.1148/radiology.194.3.7862961
- Davies MA, TerBrugge K, Willinsky R, Coyne T, Saleh J, Wallace C. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg 1996:85;830-7. https://doi.org/10.3171/jns.1996.85.5.0830
- Alatakis S, Koulouris G, Stuckey S. CT-demonstrated transcalvarial channels diagnostic of dural arteriovenous fistula. AJNR 2005:26;2393-6.
- Meckel S, Lovblad KO, Abdo G, San Millan Ruiz D, Delavelle J, Radue EW, Rufenacht DA, Wetzel AG. Arterialisation of cerebral veins on dynamic MDCT angiography: a possible sign of a dural arteriovenous fistula. AJR 2005:184;1313-6. https://doi.org/10.2214/ajr.184.4.01841313
- Houdart E, Saint-Maurice JP, Chapot R, Ditchfield A, Blanquet A, Lot G, Merland JJ. Transcranial approach for venous embolisation of dural arteriovenous fistulas. J Neurosurg 2002:97;280-6. https://doi.org/10.3171/jns.2002.97.2.0280
- Söderman M, Edner G, Erikson K, Karlsson B, Räahn T, Ulfarsson E, Andersson T. Gamma knife surgery for dural arteriovenous shunts: 25 years of experience. J Neurosurg 2006:104;867-75. https://doi.org/10.3171/jns.2006.104.6.867


