Among other pragmatic observations, the Hippocratic teachings state that “sleep and watchfulness, both of them when immoderate constitute disease”. Only four things go wrong with sleep – my patients sleep too much (hypersomnia), too little (insomnia), things go bump in the night (parasomnia/restless legs) and they sleep at the wrong time (circadian rhythm disorder). Primary sleep disorders are all common, 10% of UK men over 40 have obstructive sleep apnoea, 5% have troublesome insomnia, at least 2-3 % of adults have some form of parasomnia and the prevalence figures for all these conditions double for those over 65 or those with severe psychiatric disease. However, symptoms are often attributed to other daytime conditions rather than thinking of problems within sleep itself.
The enjoyment from sleep clinics comes from most patients having conditions that are easy to diagnose and treat, often with significant improvement in quality of life and other long term conditions. Possibly the lack of sleep medicine as an accredited sub-speciality in the UK is the reason that the average medical student receives little or no sleep medicine education. Even neurologists and psychiatrists have limited exposure to sleep disorders. This is despite the immediate and chronic impact on all aspects of brain function after sleep disturbance of any cause. In particular consolidation of new memories (separate to a simple decrease in attention) and normal mood are the key things we really need sleep for. Aspects of physical health such as hypertension, diabetes, obesity, longevity all suffer too.
So this series of articles tries to highlight sleep problems seen in general neurology and rehabilitation clinics and a simple approach to tests and treatment. Those of us who see patients with Parkinson’s Disease at any stage already know that poor sleep is a prominent and early non-motor symptom but an overview of the clinical presentation of different sleep problems, research updates and a simple guide to therapy is within this first article.
By far the commonest cause of significant daytime sleepiness is sleep apnoea, obstructive from obesity and central largely from the pain clinic (look up the simple and evidence based STOPbang questionnaire for those over 40 who are sleepy).
Narcolepsy is different with patients truly “seized by somnolence” and the exciting advances in both understanding of the molecular biology and newer therapies available will be described by Dr Reading.
Distinguishing the bumps in the night can be challenging, the patient has good reason to be an unreliable witness so Dr Dennis will explain the key aspects of history and management for those with nocturnal seizures.
Those with traumatic brain injury have many long term problems and also many reasons to have both acute and chronic sleep disturbance and a framework for diagnosis and management will be provided by Dr Singh.
Finally circadian rhythm disorders have been on the rise since the Industrial Revolution (the real start of shift work) rapidly followed by the electric light bulb and its immediate ability to disrupt 50,000 years of evolutionary biology whereby every aspect of physical and mental health evolved around a 24 hour light dark cycle. Our cellular clocks are aligned by the master time keeper within the hypothalamus – the suprachiasmatic nucleus. More severe circadian rhythm disorders are increasingly recognised as part of the molecular clockwork of severe psychiatric illness rather than simply a consequence of lack of employment and decreased activity, so spotting and fixing broken clocks will be our final article.
Dr Kirstie Anderson, Section Editor.
Sleep and Parkinson’s disease
“…the sleep becomes much disturbed. The tremulous motion of the limbs occurs during sleep, and augment until they awaken the patient, and frequently with much agitation and alarm…”
Sleep disturbance has long been recognised as an integral component of Parkinson’s Disease (PD), as evidenced by the above excerpt from James Parkinson’s seminal 1817 paper, “An Essay on the Shaking Palsy”. While his description seems to allude, counterintuitively, to tremor worsening during sleep, it is plausible that instead Parkinson was describing one or more of the primary sleep disorders which are now known to be associated with Parkinson’s Disease: REM sleep behaviour disorder and/or restless legs syndrome.
Sleep dysfunction is a salient non-motor complaint afflicting 42-98% of patients with PD,1 but for decades has been something of a “Cinderella” symptom in terms of the attention it garnered, both in the clinic room and in research literature. This is slowly changing and in this review, we will attempt to describe some of the common sleep disturbances encountered by PD patients in the pre-motor, early and advanced phases of the disease, with a focus on clinical management, as well as highlighting some of the emerging research themes in the field. As an important preface, it is essential that any evaluation of sleep in a patient with PD should consider the contribution of motor symptoms such as nocturnal hypokinesia, dystonia or dyskinesia as well as non-motor symptoms of pain, nocturia and sialorrhoea; and the neurologist should be vigilant for symptoms of depression. A thorough analysis of the patient’s medication regime is also warranted.
REM sleep behaviour disorder (RBD)
RBD, now widely recognised in clinical practice as a hallmark of the α-synucleinopathies, was first described by Schenck only thirty years ago.2 This syndrome is characterised by dream enactment behaviour; in normal circumstances, complex inhibitory neuronal circuitry results in skeletal muscle paralysis during REM sleep. Aberrant α-synuclein aggregates interfere with brainstem control of this process, leading to disinhibition of spinal muscles – it is now recognised that this process can occur decades before the evolution of motor parkinsonism.3
Clinically, the patient’s bed-partner will typically witness purposeful, often violent, movements which can result in injury to either the patient or the bed-partner. Loud vocalisation usually accompanies the motor phenomena. The patient can usually be roused easily, and will classically describe dreams with violent or threatening themes. Interestingly, the aggressive nature of the dream enactment is often at odds with the patient’s waking personality. RBD tends to manifest most reliably in the second half of the night, since the proportion of time spent in REM sleep accumulates overnight. A careful clinical history should be taken to exclude mimics of the condition – most notably non-REM parasomnias (history of earlier life sleepwalking; symptoms occurring earlier in the night; patient often rises from bed with eyes open, but is difficult to rouse and will have little or no recall if awoken; can leave the bedroom), periodic limb movements in sleep (PLMS) (a non-REM phenomenon in the early hours of sleep, with slow rhythmic dorsiflexion of the great toe and ankle and flexion of the knee, these movements can be large amplitude), or rarely dream-enactment symptoms generated in the context of a sleep-related breathing disorder such as severe obstructive sleep apnoea (OSA); these patients would often have other symptoms of daytime sleepiness. A definitive diagnosis of RBD requires polysomnographic (PSG) evidence of REM sleep without atonia (see Figure 1). In the general population, the prevalence is estimated at 0.5%, with a preponderance of older, male patients, where it can run an insidious course; although it is generally held that it is under-diagnosed in older females, who due to longer life expectancy, are more likely not to have a bed partner.4 Recognition of RBD is important in those without motor symptoms, it should alert the neurologist to monitor for the emergence of extrapyramidal signs, as it is a harbinger of neurodegenerative disease in up to 91% of patients over 14-year follow up.5 Estimates of the prevalence in patients with known PD vary from 27% in unselected cases6 to 46% in those with subjective sleep complaints7 and the syndrome can manifest at any stage of the disease. Identification of RBD in PD is important for two reasons – firstly, it portends a poorer prognosis;8,9 secondly, it can cause significant morbidity which is potentially avoidable, as it typically responds very readily to pharmacotherapy. Both melatonin (3-12mg) and clonazepam (0.5-1.0mg) are effective treatments for RBD – most centres now use the former as first line therapy given its equivalent efficacy, with a much more favourable side effect profile.10 It is important to note that many antidepressant treatments (ADTs) are associated with some dream enactment, which usually remits when the ADT is withdrawn; however an emerging literature suggests that in some cases, the ADT unmasks an underlying incipient neurodegenerative disease, manifesting as prodromal depression and RBD.11
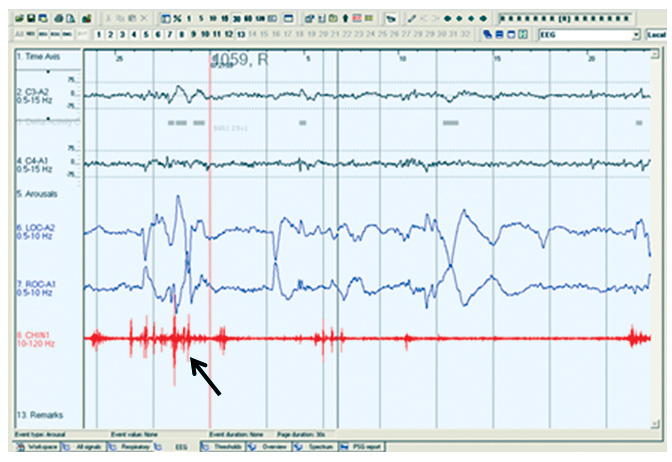
Restless legs syndrome (RLS) and periodic limb movements in sleep (PLMS)
RLS is a common neurological disorder, which is a clinical diagnosis made on the basis of fulfilling certain essential criteria. These criteria can be summarised as an urge to move the legs, accompanied by an unpleasant feeling in the legs, which is induced or exacerbated by inactivity; these symptoms exhibit a diurnal pattern and are partially or wholly relieved by movement. Other medical or behavioural factors must be excluded. The recent revision of these criteria acknowledges that an episodic variant exists.12 PLMS is a sleep-related movement disorder, defined by criteria laid out by the American Academy of Sleep Medicine, which stipulate the frequency and duration of the limb movements captured on PSG. Approximately 80% of patients with RLS exhibit PLMS,13 although the reciprocal relationship is not quite as robust. As RLS responds to dopaminergic therapy, a common pathophysiology with PD has often been postulated, although neurodegenerative features are not seen at autopsy in RLS.14 Methodological differences have led to the publication of conflicting data relating to prevalence of RLS in PD cohorts, but there does appear to be an excess in PD versus age-matched healthy controls.15 Recently, severe RLS in middle aged to older men has been associated with risk of subsequent development of PD,16 but in general RLS tends to follow the onset of parkinsonism rather than precede it.17 PLMS are frequently recognised in PD, even in the absence of symptomatic RLS, and are important to identify as they are associated with poorer subjective sleep and poorer quality of life.18 A number of studies have also highlighted a relationship between PLMS and PD severity.18,19 An important clinical point is that PLMS can vary significantly from night to night, hence a negative overnight PSG may not refute the diagnosis in the setting of a convincing history; this difficulty can be overcome by serial domiciliary lower limb actigraphy over a number of consecutive nights.
The phenotype of RLS in PD appears to differ somewhat from idiopathic RLS. PD patients develop RLS at an older age than the idiopathic group, and have a far lower rate of family history of RLS.20 It is important to recognise the possibility of RLS mimics in the PD population; various motor and sensory phenomena, particularly in the “OFF” state may at first glance appear to represent RLS symptomatology, but a rigorous application of the IRLSSG criteria will typically distinguish RLS from these. It is also important to be aware of the propensity of certain adjunctive treatments in PD to unmask or exacerbate RLS; many antidepressants,21 in particular mirtazapine22 (commonly used in the PD cohort) have been implicated; there are also data demonstrating worsening of RLS with the administration of exogenous melatonin.23 The relationship between RLS and dopaminergic therapy in PD is ambiguous; although an evidence-based treatment in idiopathic RLS, some authors have suggested that long duration of dopaminergic therapy in PD is deleterious from an RLS perspective.14 Akin to PLMS, RLS in PD is associated with increased non-motor symptoms, poorer quality of life and greater daytime fatigue,24 and as such, should be recognised and treated expeditiously. In those with ferritin levels below 50mcg/l, iron supplementation should be instituted. Consideration should be given to the addition of a small evening dose of a dopamine agonist if this is not already a component of their PD regimen. The other line of treatment of particular utility in this cohort is an alpha-2-delta agonist such as gabapentin or pregabalin.
Excessive daytime somnolence (EDS)
EDS is a symptom frequently volunteered by PD patients or their carers in the clinic. Whilst the evaluation of EDS can be confounded by the use of dopaminergic and other psychotropic drugs with disease progression, data from incident cohorts25 have identified greater rates of daytime napping in early PD patients than controls, and EDS is also diagnosed more frequently in de novo drug-naïve patients than controls.26 Larger cross-sectional studies have reported EDS in 51% of functionally independent, non-demented PD subjects.27 Higher sleepiness scores are associated with a less favourable motor phenotype28 and sleepiness indices worsen with time,29 and as cognitive impairment and dementia ensue.30
EDS is readily quantified in clinic by means of the self-completed Epworth Sleepiness Scale, wherein patients rate the likelihood of falling asleep in eight everyday situations; a score greater than 10 is considered abnormal. Clinicians should be vigilant for EDS amongst PD patients, particularly those that drive. Complaints of daytime sleepiness should prompt a thorough review of nocturnal symptoms which may predispose to fragmented sleep and consequent EDS. In particular, obstructive sleep apnoea and other sleep disordered breathing (SDB) conditions should not be missed as a common and reversible cause of EDS. OSA is at least as common in PD as in age-matched controls;31 furthermore PD patients tend to sleep in the supine position more frequently than controls,32 which will worsen any underlying SDB. Suspicion of SDB in a PD patient warrants at least overnight home oximetry studies to determine if this is contributory to excessive sleepiness. Continuous positive airways pressure (CPAP) should be instituted where OSA is identified.
Nocturia is another under-recognised nocturnal symptom in PD with significant potential to fragment sleep and if identified, warrants further assessment, as it will often respond in appropriate cases to a selective urinary antispasmodic. Similarly, troublesome sialorrhoea can respond well to low-dose tricyclic agents or clonidine. Motor symptoms affecting sleep should prompt a review of the timing and nature of dopaminergic treatments – addition of a controlled release levodopa preparation at bedtime can often ameliorate these significantly.
In the absence of a clear secondary cause of EDS, subjective improvements can be seen with modafinil (although these were not borne out on objective measures of sleep latency in trials);33 headaches and nausea are common adverse effects and the prescriber should be mindful of potential deleterious cardiovascular effects in an elderly cohort.
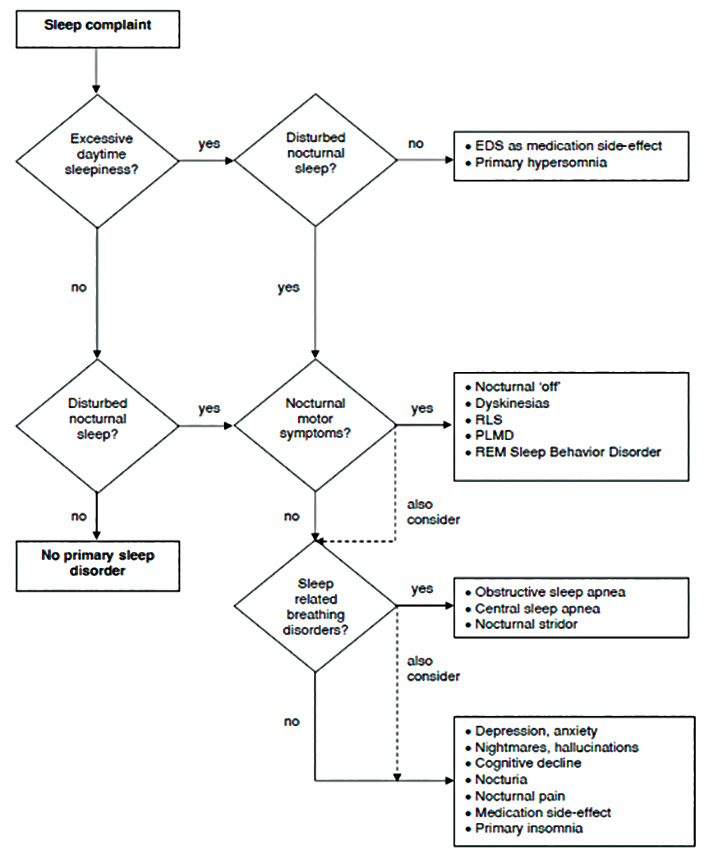
Class I evidence for many of the above strategies is lacking, but many guidelines encompass these approaches. Figure 2 provides a useful algorithm for an approach to EDS and other sleep symptoms in PD.34
Research directions
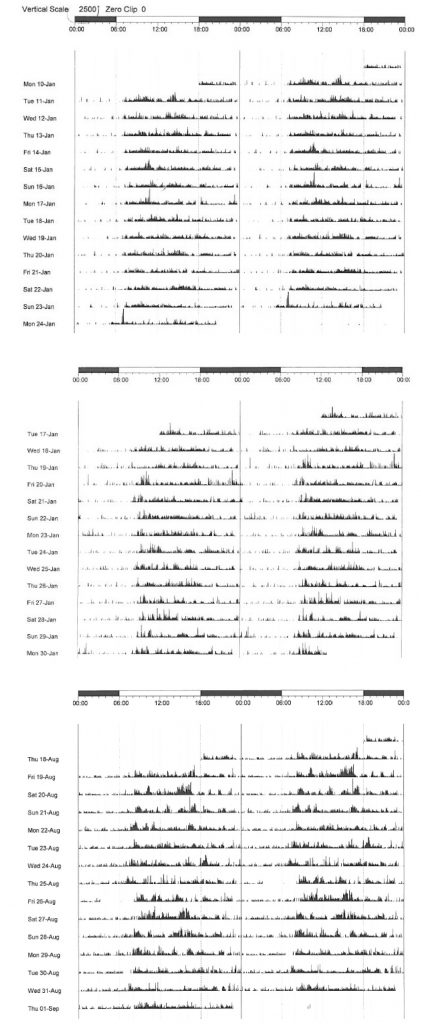
Sleep dysfunction is increasingly attracting attention in the realms of neurodegenerative research. The observation that sleep deprivation in transgenic mice leads to increased production of β-amyloid plaque35 has prompted much interest in sleep in Alzheimer’s disease (AD) research, and there is compelling evidence that sleep facilitates β-amyloid clearance via the recently described “glymphatic” system.36 Sleep fragmentation has been implicated in increasing the risk of AD37 and micro-architectural sleep alterations are well recognised in PSG studies of the condition.38 Similarly, there are emerging data pinpointing early alterations in sleep structure in PD as a potential biomarker for the later development of PD dementia.39 A burgeoning body of evidence from longitudinal, prospective cohorts indicts poor-quality sleep as a risk factor for dementia.40 In our centre, unfavourable actigraphic measures of sleep obtained at baseline in an incident PD cohort eloquently predicted the decline into cognitive impairment and subsequent PD dementia (manuscript in preparation) (see Figure 3). The debate continues as to whether poor sleep initiates or hastens neurodegeneration, or whether disrupted sleep is merely a hitherto under-recognised prodromal feature. The possibility that poor sleep either accelerates or triggers neurodegeneration raises the tantalising possibility that early intervention with strategies to consolidate sleep and/or stabilise circadian rhythm may be neuroprotective, and will undoubtedly form the basis for further research.
Learning points:
- Patients who develop REM sleep behaviour disorder (RBD) in the absence of other neurological symptoms should be monitored for the evolution of extrapyramidal signs; up to 90% will develop a neurodegenerative disease.
- Those patients who do manifest RBD as a component of their PD tend to have a poorer prognosis from both a motor and cognitive perspective.
- Melatonin is as efficacious as clonazepam for RBD and is better tolerated.
- Antidepressants can provoke RBD, which is usually reversible upon withdrawal.
- Restless legs syndrome (RLS) is common in PD and should be treated to reduce the impact on sleep disturbance and quality of life. The clinician should be vigilant for the tendency of drugs such as mirtazapine or melatonin to provoke or exacerbate RLS.
- Excessive daytime somnolence in PD should be recognised as a debilitating symptom and secondary causes such as obstructive sleep apnoea should be sought and treated.
References
- Ratti PL, Negre-Pages L, Perez-Lloret S, et al. Subjective sleep dysfunction and insomnia symptoms in Parkinson’s disease: Insights from a cross-sectional evaluation of the French CoPark cohort. Parkinsonism Relat Disord 2015;21:1323-1329.
- Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep 1986;9:293-308.
- Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med 2013;14:744-748.
- Howell MJ, Schenck CH. Rapid Eye Movement Sleep Behavior Disorder and Neurodegenerative Disease. JAMA Neurol 2015;72:707-712.
- Iranzo A, Fernandez-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One 2014;9:e89741.
- Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson’s disease over time. J Neurol Neurosurg Psychiatry 2008;79:387-391.
- Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology 2011;77:1048-1054.
- Anang JB, Gagnon JF, Bertrand JA, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 2014;83:1253-1260.
- Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison With Other Phenotypes. JAMA Neurol 2015;72:863-873.
- McCarter SJ, Boswell CL, St Louis EK, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med 2013;14:237-242.
- Postuma RB, Gagnon JF, Tuineaig M, et al. Antidepressants and REM sleep behavior disorder: isolated side effect or neurodegenerative signal? Sleep 2013;36:1579-1585.
- Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med 2014;15:860-873.
- Montplaisir J, Boucher S, Nicolas A, et al. Immobilization tests and periodic leg movements in sleep for the diagnosis of restless leg syndrome. Mov Disord 1998;13:324-329.
- Rijsman RM, Schoolderman LF, Rundervoort RS, Louter M. Restless legs syndrome in Parkinson’s disease. Parkinsonism & Related Disorders 2014;20:S5-S9.
- Krishnan PR, Bhatia M, Behari M. Restless legs syndrome in Parkinson’s disease: a case-controlled study. Mov Disord 2003;18:181-185.
- Wong JC, Li Y, Schwarzschild MA, Ascherio A, Gao X. Restless legs syndrome: an early clinical feature of Parkinson disease in men. Sleep 2014;37:369-372.
- Suzuki K, Miyamoto M, Miyamoto T, Hirata K. Restless Legs Syndrome and Leg Motor Restlessness in Parkinson’s Disease. Parkinsons Dis 2015;2015:490938.
- Covassin N, Neikrug AB, Liu L, et al. Clinical correlates of periodic limb movements in sleep in Parkinson’s disease. J Neurol Sci 2012;316:131-136.
- Young A, Home M, Churchward T, Freezer N, Holmes P, Ho M. Comparison of sleep disturbance in mild versus severe Parkinson’s disease. Sleep 2002;25:573-577.
- Lee JE, Shin HW, Kim KS, Sohn YH. Factors contributing to the development of restless legs syndrome in patients with Parkinson disease. Mov Disord 2009;24:579-582.
- Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry 2005;58:510-514.
- Fulda S, Kloiber S, Dose T, et al. Mirtazapine provokes periodic leg movements during sleep in young healthy men. Sleep 2013;36:661-669.
- Whittom S, Dumont M, Petit D, et al. Effects of melatonin and bright light administration on motor and sensory symptoms of RLS. Sleep Med 2010;11:351-355.
- Neikrug AB, Maglione JE, Liu L, et al. Effects of sleep disorders on the non-motor symptoms of Parkinson disease. J Clin Sleep Med 2013;9:1119-1129.
- Prudon B, Duncan GW, Khoo TK, Yarnall AJ, Anderson KN. Primary sleep disorder prevalence in patients with newly diagnosed Parkinson’s disease. Mov Disord 2014;29:259-262.
- Tholfsen LK, Larsen JP, Schulz J, Tysnes OB, Gjerstad MD. Development of excessive daytime sleepiness in early Parkinson disease. Neurology 2015;85:162-168.
- Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. Jama 2002;287:455-463.
- Breen DP, Williams-Gray CH, Mason SL, Foltynie T, Barker RA. Excessive daytime sleepiness and its risk factors in incident Parkinson’s disease. J Neurol Neurosurg Psychiatry 2013;84:233-234.
- Gjerstad MD, Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP. Excessive daytime sleepiness in Parkinson disease: is it the drugs or the disease? Neurology 2006;67:853-858.
- Goldman JG, Ghode RA, Ouyang B, Bernard B, Goetz CG, Stebbins GT. Dissociations among daytime sleepiness, nighttime sleep, and cognitive status in Parkinson’s disease. Parkinsonism Relat Disord 2013;19:806-811.
- Peeraully T, Yong MH, Chokroverty S, Tan EK. Sleep and Parkinson’s disease: a review of case-control polysomnography studies. Mov Disord 2012;27:1729-1737.
- Sommerauer M, Werth E, Poryazova R, Gavrilov YV, Hauser S, Valko PO. Bound to supine sleep: Parkinson’s disease and the impact of nocturnal immobility. Parkinsonism Relat Disord 2015;21:1269-1272.
- Zesiewicz TA, Sullivan KL, Arnulf I, et al. Practice Parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010;74:924-931.
- Louter M, Aarden WC, Lion J, Bloem BR, Overeem S. Recognition and diagnosis of sleep disorders in Parkinson’s disease. J Neurol 2012;259:2031-2040.
- Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009;326:1005-1007.
- Tarasoff-Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 2015;11:457-470.
- Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 2013;36:1027-1032.
- Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev 2015;19:29-38.
- Latreille V, Carrier J, Lafortune M, et al. Sleep spindles in Parkinson’s disease may predict the development of dementia. Neurobiol Aging 2015;36:1083-1090.
- Blackwell T, Yaffe K, Laffan A, et al. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep 2014;37:655-663.

