Introduction
The term acoustic neuroma is a misnomer on two accounts. These benign tumours usually arise from the Schwann cells at the myelination-glial junction of the superior division of the Vestibular Nerve. As they grow from the internal auditory canal into the cerebello-pontine (CP) angle, they invaginate the arachnoid and remain covered by it regardless of size [1]. With increasing use of MRI the detection of vestibular schwannomas has increased to around 1/100000 per year [2]. This represents around 6-10% of all intracranial tumours.
The loss of a tumour suppressor gene on the long arm of chromosome 22 has been found in 40% of cases [3]. The presence of bilateral vestibular schwannomas is diagnostic for neurofibromatosis II.
Progressive hearing loss (59%), tinnitus (13%) and imbalance (10%) are the most common presenting symptoms caused by vestibular schwannomas [4]. These are followed by sudden hearing loss (8%), facial numbness (3%), headaches (1%) and visual disturbance (1%). Large tumours (>3cm) can cause brainstem compression and obstructive hydrocephalus. Rarely communicating hydrocephalus secondary to elevated CSF protein can occur. Physical signs include sensorineural hearing loss, an attenuated corneal reflex, facial hypoesthesia, diplopia and cerebellar signs. Facial weakness is a rare finding on presentation.
Investigations
MRI with gadolinium contrast is the imaging investigation of choice (Figure 1). Vestibular schwannomas enhance and can be differentiated from CPA meningiomas as they expand the porus acousticus, lack a dural tail and have an acute rather than an obtuse angle at the petrous face. The CISS sequence is also useful to delineate the course of the neurovascular bundle in the CP angle. A pure tone audiogram (Figure 2) and speech discrimination testing (where appropriate) determine the level of hearing improvement and help judge the value of attempting hearing preservation treatment.
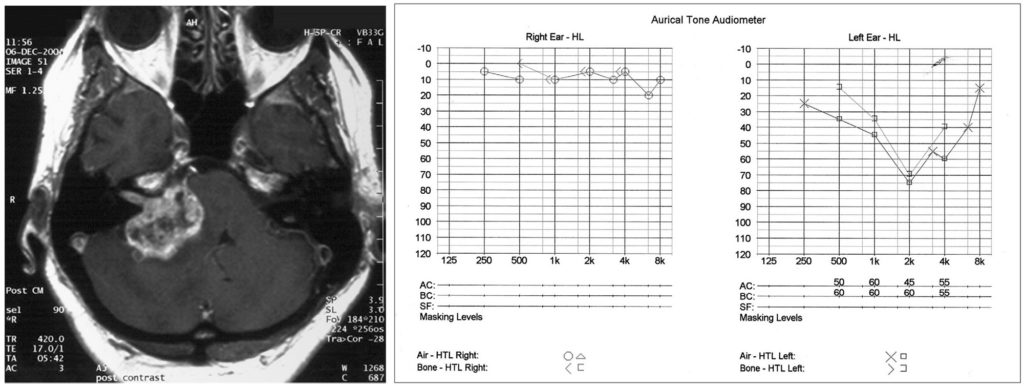
Figure 2 (right): Pure tone audiogram demonstrating left sided mid frequency sensorineural hearing loss, characterised by impaired bone and air conduction.
Management
Management of patients with vestibular schwannomas is determined by several factors including symptoms, patient age, co-morbidity, tumour size, rate of growth and patient preference.
Treatment options are:
- Conservative management with planned tumour
surveillance - Stereotactic radiosurgery
- Microsurgery
Conservative management
The frequent use of MRI scans has increased the detection of small asymptomatic tumours and small intracanalicular tumours presenting with unilateral hearing loss. A ‘watch, wait and rescan’ approach to these lesions is often adopted as intervention does carry risk.
Analysis of 13 studies involving 903 patients managed conservatively (mean tumour size of 10mm diameter) shows that 49% of tumours showed growth on subsequent radiological imaging, 47% showed no growth while 4% regressed. The average growth rate was 1.87mm/year [5]. Tumour growth was reported as high as 30mm/year in one patient. 20% of 804 patients managed conservatively subsequently required intervention mostly as a result of progression of symptoms.
In elderly patients with co-morbidity, the conservative approach may be most appropriate due to the risks of intervention. The optimal frequency of repeat imaging is not clearly defined. We monitor patients with interval scans at 6 and 12 months after presentation and then annually.
Stereotactic radiosurgery
Leksell described Gamma-knife Radiosurgery for Acoustic Neuromas in 1971 [6]. It has been used for the treatment of small to medium sized tumours and incompletely resected lesions. The rapid return to normal activity and avoidance of an open procedure are attractive alternatives to microsurgery. The treatment aims to prevent further growth and maintain neurologic function.
Of 162 consecutive patients followed up for a minimum of 5 years in Pittsburgh, tumour size diminished in 61.7%, remained static in 32.7% and showed slow growth in 5.6%. Normal facial and trigeminal nerve function was evident in 79% and 73% respectively at follow-up [7]. The Sheffield group reported a tumour control rate of 92% among 234 tumours treated over a 4-year period with only 3% of patients requiring microsurgery [8].
Ionising radiation is associated with a risk of malignant transformation in benign tumours. The frequency of this complication is difficult to ascertain. The Sheffield group has reported 1 case of malignant transformation among 800 patients with vestibular schwannomas treated by Stereotactic radiosurgery [8].
Microsurgery
Microsurgery is the treatment of choice for large vestibular schwannomas and is worthy of consideration in patients with small and medium sized tumours. Three surgical approaches to the CP angle may be adopted. Each has merits and drawbacks.
- Retrosigmoid approach
- Translabyrinthine approach
- Middle fossa approach
Retrosigmoid approach
A craniectomy just posterior to the sigmoid sinus with retraction of the cerebellum provides good visualisation of the CP angle component of a vestibular schwannoma [1]. Intra-operative facial nerve monitoring helps to identify the facial nerve as it is very often stretched out over the anterior aspect of the tumour. Limitations of this approach include the retraction of the cerebellar hemisphere, which may be excessive especially with larger tumours. The approach provides poor access to small laterally located intracanalicular tumours, unless careful drilling of the petrous bone is performed. It is the approach of choice when attempting hearing preservation.
Translabyrinthine approach
This is the shortest, most direct approach to the CP angle but always results in a dead ear. It is the approach of choice for laterally located intracanalicular tumours where hearing preservation is not required as well as for large tumours minimising cerebellar retraction [9]. It involves an extended mastoidectomy and labyrinthinectomy. The facial nerve is exposed at the fundus of the internal auditory canal along with the canalicular portion of the tumour. The brainstem end of the facial nerve is then identified enabling vigilant protection during resection of the tumour bulk using a cavitating ultrasonic surgical aspirator (CUSA).
Middle fossa approach
This approach has not been universally adopted. It is reported to be useful for small intracanalicular tumours where hearing preservation is desired. It involves an extradural approach in the floor of the middle fossa with retraction of the temporal lobe to expose the petrous temporal bone [10]. Drawbacks of this approach are increased risk of epilepsy from temporal lobe retraction, poor access to the posterior fossa and risk of injury to the facial nerve at the level of the geniculate ganglion.
Results of microsurgery
The object of vestibular schwannoma surgery is the total removal of the neoplastic lesion with minimal morbidity and mortality. Objective recordings of cranial nerve function, CSF leak rates, meningitis, and quality of life assessments can be used to assess morbidity. Extent of tumour removal can be determined intraoperatively, and recurrence can be monitored with MRI scans.
Review of data from over 5000 patients in 16 studies who underwent surgical tumour removal between 1972 and 1999 revealed an average of 96% (93% – 100%) total tumour removal with a recurrence rate of 1.8% [5].
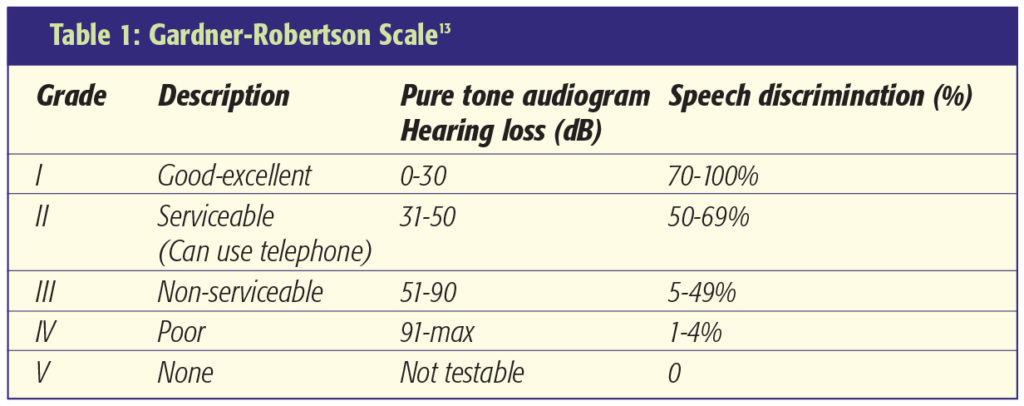
A series of 179 patients who underwent microsurgery reports the rates of facial nerve preservation with respect to the size of tumour. Excellent results occurred in 96% of small tumours (<2cm), 74% of medium tumours (2.0 – 3.9cm) and 38% of large tumours (4cm). Serviceable hearing was achieved in 48% of small tumours and 25% of medium tumours [12]. Hearing preservation is rarely an objective in patients with large tumours.
Facial nerve function
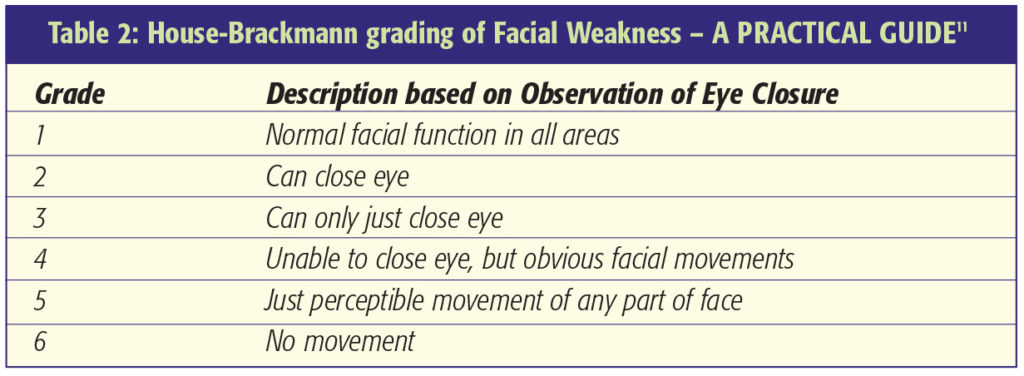
Facial Nerve Function is assessed using the House-Brackmann Scale [11]. This grades facial weakness from normal (I) through to total paralysis (VI) (Table 2). The extracapsular dissection of the tumour capsule from the surrounding neurovascular structures should be done along the tumour capsule-arachnoid interface and not the interface between the arachnoid and neurovascular structures [1]. This ensures protection of the neurovascular structures, especially the facial nerve, by a layer of arachnoid. Some surgeons advocate incomplete resection of large tumours with adjuvant stereotactic radiosurgery to attempt reduced facial nerve morbidity. Loss of the facial nerve can be repaired by use of an interposition cable graft using the great auricular or sural nerve. Facial reanimation techniques can be performed at a later stage if considered appropriate. Decreased lacrimation and corneal exposure both predispose to keratitis. Eye lubrication, taping the eye shut at night, Botulinum injection, tarsorrhaphy and gold eyelid weights are all techniques worthy of consideration in patients with facial weakness where the cornea is at risk.

Summary
The management of patients with vestibular schwannomas is by no means clear-cut and many factors need to be taken into account. Microsurgery offers the best tumour control but has a considerable complication rate. The size, site and aim for hearing preservation would determine the route taken. Over the past 4 decades, radiosurgery has become a viable alternative for small to medium sized tumours and offers effective tumour control. Tumour surveillance with targeted intervention has a role to play in elderly patients and some patients with asymptomatic lesions. The frequency of repeat imaging is empirical. At our institution, patients have six monthly scans for the first year decreasing to annually or even longer depending on the rate of growth with a low threshold to rescan if new symptoms arise prevails.
References
- Yasargil MG, et al. Microneurosurgery: Microsurgery of CNS Tumours. Thieme Publishing Group – October 1995.
- Tos M, Charabi S, Thomsen J, Korol HW, Nutik SL, Smith R. Incidence of acoustic neuromas. Ear Nose Throat J. 1992Sep;71(9):391-3. https://doi.org/10.1177/014556139207100907
- Irving RM, Moffat DA, Hardy DG, et al. A molecular, clinical, and immunohistochemical study of vestibular schwannoma. Otolaryngol Head Neck Surg. 1997Apr;116(4):426-30.
- Moffat DA, Jones SEM, Mahendran R, et al. Referral patterns in vestibular schwannomas – 10 years on. Clin Otolaryngol Allied Sci. 2004Oct;29(5):515-7. https://doi.org/10.1111/j.1365-2273.2004.00854.x
- Yamakami I, Uchino Y, Kobayashi E, Yamaura A. Conservative Management, gamma-knife radiosurgery, and microsurgery for acoustic neurinomas: A systematic review of outcome and risk of three therapeutic options. Neurological Research; Oct2003;25,7;ProQuest Medical Library pg. 682-90. https://doi.org/10.1179/016164103101202075
- Leksell L. A note on the treatment of acoustic tumours. Acta Chir Scand. 1971;137(8):763-5.
- Kondziolka D, Lunsford LD, Mc Laughlin MR et al. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med 1998Nov12;339(20):1426-33. https://doi.org/10.1056/NEJM199811123392003
- Rowe JG, Radatz MWR, Walton L, Hampshire A, Seaman S, Kennedy AA. Gamma knife stereotactic radiosurgery for unilateral acoustic neuromas. J Neurol Neurosurg Psychiatry. 2003Nov;74(11):1536-42. https://doi.org/10.1136/jnnp.74.11.1536
- Lanman TH, Brackmann DE, Hitselberger WE, Subin B. Report of 190 consecutive cases of large acoustic tumors (vestibular schwannoma) removed via the translabyrinthine approach. J Neurosurg. 1999Apr;90(4):617-23. https://doi.org/10.3171/jns.1999.90.4.0617
- Gjuric M, Wigand ME, Wolf SR. Enlarged middle fossa vestibular schwannoma surgery: experience with 735 cases. Otol Neurotol. 2001Mar;22(2):223-30. https://doi.org/10.1097/00129492-200103000-00019
- House W F, Brackmann D E. Facial Nerve Grading System. Otolaryngol Head Neck Surg 93:184-93,1985. https://doi.org/10.1177/019459988509300202
- Gormley WB, Sekhar LN, Wright DC et al. Acoustic neuromas: results of current surgical management. Neurosurgery. 1997Jul;41(1):50-8. https://doi.org/10.1097/00006123-199707000-00012
- Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988 Jan-Feb;97(1):55-66. https://doi.org/10.1177/000348948809700110

