Abstract
Migraine and epilepsy account for more than 40% of neurology outpatients and are leading causes of disability [1]. They often co-exist and can be confused, because of shared clinical features. The borderlands and links between migraine and epilepsy have fascinated neurologists for centuries, and unresolved questions remain. Greater understanding of the relationship between migraine and epilepsy may give insight into shared mechanisms. It is already clear that treating co-existing migraine is an important therapeutic opportunity and may improve epilepsy [2].
Definitions
There are no specific biomarkers for epilepsy or migraine. Outpatient studies suggest the diagnosis of epilepsy is accurate in about 2/3 of cases [3], and migraine definitions are subjective. Revised definitions of epilepsy emphasise the chance of seizure recurrence, combining clinical and investigation features [4]. The International Headache Society definition aims for clarity, presenting migraine as an episodic headache lasting 4-72 hours. The definition requires two of the following: worsened by movement, unilateral, throbbing, moderate or severe; and either nausea and/or vomiting or photophobia and phonophobia [5]. This rigid definition misses much of the migraine encountered in clinical practice [6]. The distinctions between migraine and tension headache are also blurred, meaning that some migraine associated with epilepsy is mislabelled as tension headache. Migraine may recur with no headache or may simply be an aura, and pain may be in the abdomen or limbs, particularly in children [6,7]. Earlier definitions of migraine were more fluid, and arguably more reflective of real life – “recurrent attacks of headache widely varied in intensity, frequency and duration…commonly unilateral in onset; are usually associated with anorexia, and sometimes with nausea and vomiting; and some are proceeded by, or associated with, conspicuous sensory, motor, and mood disturbances; and are often familial” [8]. A key feature of migraine is hypersensitivity to sensory stimuli between as well as during episodes. There may be autonomic, motor, cognitive, psychic and sensory features as well as the headache [6].
Shared clinical features
Similarities between migraine and epilepsy in triggers, prodrome, aura, features of the ictus, and treatment demand careful analysis to avoid confusion.
Triggers
Alcohol withdrawal is a trigger for an estimated one-fifth of seizures, usually occurring 6-12 hours after imbibing (usually >7 U) and predominantly in genetic generalised epilepsy [9]. Alcohol-induced migraine (veisalgia or “hangovers”) as well as acute sensitivity, particularly for red wine, are well recognised in migraineurs [10]. For migraine and epilepsy, sleep and food deprivation, as well as stress and relaxation may be individual triggers. With regard to visual stimuli, photophobia is a feature in 80% of people with migraine in at least some attacks [6]. In epilepsy only 5% of patients have photosensitivity or photoconvulsive seizures [11].
Prodrome
30-90% of people with migraine have a prodrome [12] manifestating heightened sensitivity to sensory stimuli. 6-40% (average 30%) of people with epilepsy report a prodrome, including heightened sensitivity, changes in cognition, mood or appetite, and a migraine headache in 8% [13].
Aura
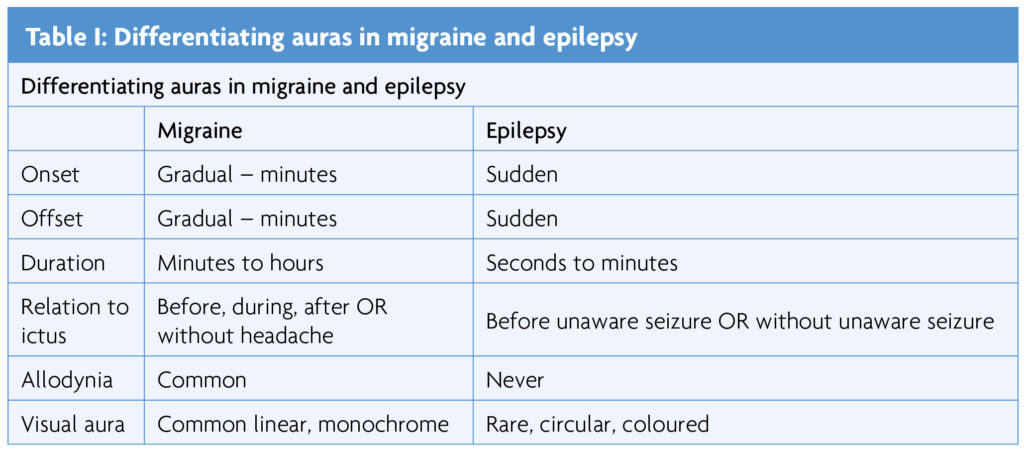
Migraine aura may occur without a headache, or before, during or after one, and may be positive (such as tingling) or negative (for example hemiplegia) [6]. In epilepsy, aura (now denoted focal aware seizures) are usually positive phenomena and occur as the seizure manifestation or at seizure onset. Some aura features are similar – this is particularly important for visual, gustatory and olfactory sensations. Duration and evolution is the most important distinction – epileptic aura are usually brief with sudden onset and offset, migraine aura usually evolve and recede, lasting many minutes [6,14].
Elementary visual hallucinations are the most common migraine aura – usually linear, monochrome, 5-30 minutes duration, with gradual onset and offset [14]. They are usually distinguished from the occipital epilepsy aura, characteristically less than 2 minutes duration, rapid in onset and offset, coloured and circular [14]. Complex visual hallucinations are rare in both migraine and epilepsy and alternative diagnoses should always be considered [6,14]. Olfactory hallucinations, usually brief and unpleasant, occur in 1-66% of those with temporal lobe epilepsy [15]. They also happen in migraine, lasting between 5 minutes and 24 hours, and may be misdiagnosed as epilepsy [16]. Similarly, brief, unpleasant elementary gustatory hallucinations are well documented in temporal lobe epilepsy but also occur in migraine. Elementary auditory hallucinations (tinnitus) are a frequent migraine aura [6]. Vestibular hallucinations (vertigo or disequilibrium) occur in 30-50% of people with migraine [17], typically lasting minutes or hours. The frequency of auditory and vestibular hallucinations in epilepsy is uncertain.
Autonomic features occur in more than 80% of migraine [5-7], usually lasting for hours, most commonly vomiting, pallor or sweating. In epilepsy they are uncommon, except in childhood occipital epilepsy and temporal lobe epilepsies. A brief epigastric aura occurs in up to 27% of people with temporal lobe epilepsy [18].
Somatosensory aura, usually tingling, is frequent in migraine. As clinical examination is normal it is often misdiagnosed as functional [6]. In migraine, head pain is present during at least some episodes in most cases. Variants include abdominal migraine or limb pain (periodic syndromes), particularly in children; but in 1-2% of adult migraine sufferers [19,20]. Ictal pain is reported in <3% of seizures [21,22], usually parietal or temporal lobe onset and most common with head, abdominal or limb pain reported, of duration from seconds to 30 minutes, and variable intensity [21]. Allodynia is a key distinguishing feature as it is not present with epilepsy, but is present in 40-60% of people with migraine [23].
Prevalence
Overall population incidence of migraine is 10%, but 50-80% in neurologists and headache specialists [24], at least partly due to heightened awareness. Migraine is primary, with a family history in about 70% [5]. The epilepsies are a collection of different conditions, occurring in 1% of the population, with established aetiology in about half, and genetic factors increasingly recognised [25]. Migraine is more common in people with epilepsy (and vice versa), estimated as 8-33% in unselected epilepsy populations with variable study design making meta-analysis problematic [2,26] and higher in those with catamenial epilepsy and migraine with aura [2]. Incidence of migraine varies with epilepsy subtype, occurring in 75% of people with occipital epilepsy [14].
Peri-ictal headache & interaction with epilepsy
There is no evidence that migraine causes epilepsy or vice versa [27] but peri-ictal headache is common and undertreated. Migraine may precede or follow a seizure, again arguing against a causal link. Migralepsy (or migraine–triggered seizures, the newer but clumsier term) [5], is described as classic visual aura followed by a seizure occurring within an hour of the aura [28]. Although controversial, it has been demonstrated electrographically in a few cases, and described convincingly in others. Marks and Ehrenberg [2] recorded the entire sequence from migraine aura to partial seizure in two patients with distinctive changes on the EEG during the migraine aura preceding the onset of an electrographic seizure. In five other patients, periodic lateralised epileptiform discharges were recorded in close temporal relation to their migraine attacks. Childhood occipital epilepsies have migrainous features at onset, and arguably represent a form of migralepsy [14]. Complicating interpretation, EEG changes occur in up to 43% of migraineurs (usually non-specific delta and theta waves, but occasionally spike-and-wave) [29]. This highlights the hazards of requesting an EEG in people investigated for syncope with co-existing migraine.
Ictal headaches are rarely described – hemicrania epileptica and epileptic headache are defined by epileptiform EEG changes [30], and as EEG is rarely recorded during migraines, the exact incidence is unknown.
Postictal headache is reported in 31-56% of patients after a tonic-clonic seizure but not after absence seizures [31,32]. 50-70% of sufferers have interictal migraine and 6% have pre-ictal headache. These headaches usually fulfil criteria for migraine [31].
Treatment of peri-ictal and inter-ictal migraine
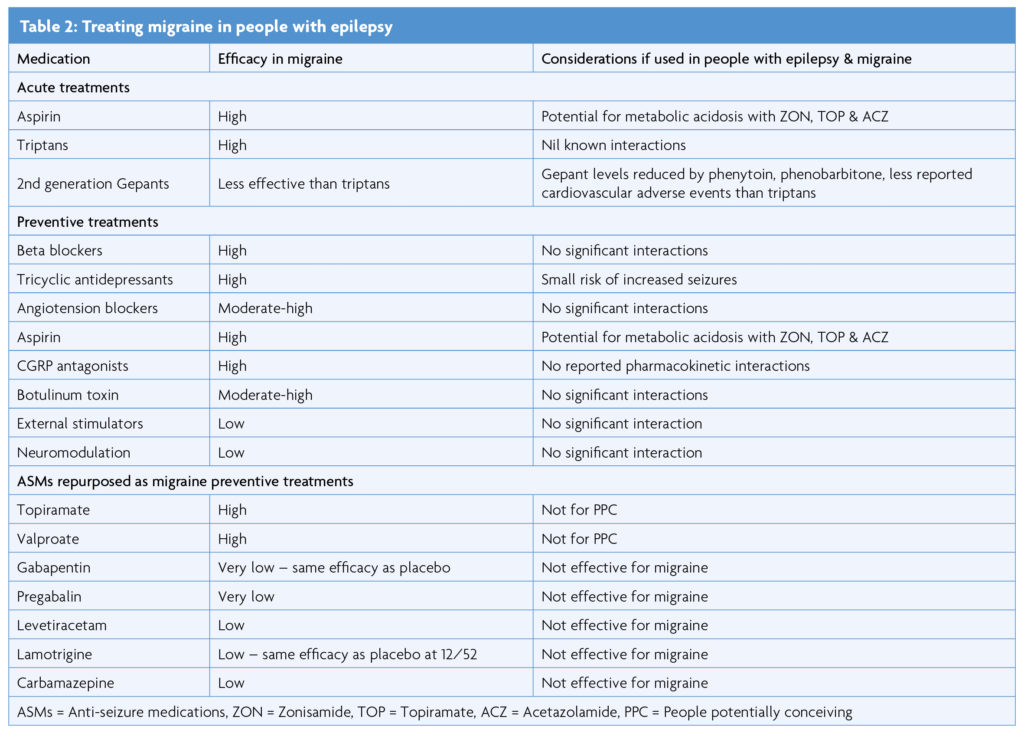
Treating migraine in people with co-existent epilepsy and migraine potentially improves both conditions. In 6/79 (8%) of patients with refractory epilepsy, adding anti-migraine treatment to ASMs improved seizure control [2].
There are no randomised controlled trials specifically investigating treatment of migraine associated epilepsy. Some antiseizure medications (ASMs) work for both migraine and epilepsy. Topiramate and valproate have class I evidence for efficacy in both conditions [33]. Of other drugs with efficacy in epilepsy, levetiracetam, lamotrigine, gabapentin and pregabalin have also been trialled for migraine but without conclusive evidence of efficacy [34]. Most of the standard migraine treatments are suitable for people with epilepsy, and considerations are summarised in the Table 2. Whether to try to treat both conditions with a single medication, or separately, is a matter of individual clinical judgement tailored to the individual’s lifestyle, co-morbidities and treatment preferences.
Shared mechanisms
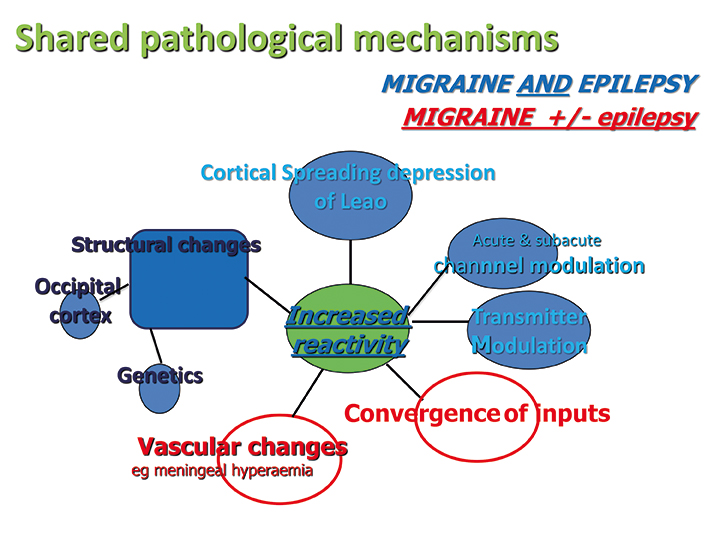
Links between migraine and epilepsy are indirect. Shared pathological factors include structural changes in the occipital cortex, genetic changes particularly channelopathies, cortical spreading depression of Leão [14,32] and acute and sub-acute channel and transmitter modulation [14]. Specific transmitter changes in the occipital cortex may have a role, with increased glutamate-to-glutamine ratio reported in women with migraine, and high neuron-to-astrocyte ratio, with these neurons containing the high glutamate-to-glutamine ratio [14,32]. Astrocytes, important in the reversal of the changes of cortical spreading depression are less in occipital cortex [14]. Trauma may cause epilepsy through glutamate receptor activation, excitotoxicity and hypersynchrony, changes which can also trigger migraine [14]. Rare monogenic channel defects produce both migraine and epilepsy [35]. Convergence of inputs and vascular changes, particularly meningeal hyperaemia are important mechanisms in migraine, little studied in epilepsy [36,37]. Analysis of the mechanisms of the two ASMs with proven efficacy in migraine does not resolve pathogenesis, due to their multiple mechanisms of activity. Topiramate changes GABA, AMPA, sodium channels and calcium channels, and sodium valproate increases GABA, enhances GABA response, increases potassium conductance and reduces neurogenic inflammation [33].
Conclusions
Careful clinical assessment usually allows differentiation of the shared features of migraine and epilepsy. Migraine is a common co-morbidity for people with epilepsy, occurring in more than 20% of people with epilepsy overall, and 75% in people with occipital lobe epilepsy [14]. It is most commonly post-ictal, but may also be prodromal, pre-ictal, or ictal. Most migraine therapy can be used safely in people with epilepsy. Pro-active treatment of co-morbid migraine reduces an unnecessary burden for people with epilepsy, and may directly improve their epilepsy [2].
References
- Angus-Leppan H, Guiloff AE, Benson K, Guiloff RJ. Navigating migraine care through the COVID-19 pandemic: an update. Journal of Neurology 2021;1-8. https://doi.org/10.1007/s00415-021-10610-w
- Marks DA, Ehrenberg BL. Migraine-related seizures in adults with epilepsy, with EEG correlation. Neurology. 1993;43(12):2476-83. https://doi.org/10.1212/WNL.43.12.2476
- Angus-Leppan H. Diagnosing epilepsy in neurology clinics: a prospective study. Seizure. 2008;17(5):431-6. https://doi.org/10.1016/j.seizure.2007.12.010
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr, Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshé SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475-82. https://doi.org/10.1111/epi.12550
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. https://doi.org/10.1177/0333102417738202
- Angus-Leppan H. Migraine: mimics, borderlands and chameleons. Pract Neurol. 2013 Oct;13(5):308-18. https://doi.org/10.1136/practneurol-2012-000502
- Angus-Leppan H, Saatci D, Sutcliffe A, Guiloff RJ. Abdominal migraine. BMJ. 2018;19;360:k179. https://doi.org/10.1136/bmj.k179
- Ad Hoc Committee on Classification of Headache. Classification of Headache. JAMA.1962;179:717-718. International Headache Society, JAMA 1962. https://doi.org/10.1001/jama.1962.03050090045008
- Hamerle M, Ghaeni L, Kowski A, Weissinger F, Holtkamp M. Alcohol Use and Alcohol-Related Seizures in Patients With Epilepsy. Front Neurol. 2018;9:401.
- Zlotnik Y, Plakht Y, Aven A, Engel Y, Am NB, Ifergane G. Alcohol consumption and hangover patterns among migraine sufferers. J Neurosci Rural Pract. 2014;5(2):128-134. https://doi.org/10.4103/0976-3147.131652
- Angus-Leppan H. Seizures & other adverse events during routine scalp EEG. Clin Neurophysiol 2007:118,22-30. https://doi.org/10.1016/j.clinph.2006.08.014
- Becker WJ. The premonitory phase of migraine and migraine management. Cephalalgia. 2012;33(13):1117-1121. https://doi.org/10.1177/0333102412437390
- Besag FMC, Vasey MJ. Prodrome in epilepsy. Epilepsy Behav. 2018 Jun;83:219-233. https://doi.org/10.1016/j.yebeh.2018.03.019
- Angus-Leppan H, Clay TA. Adult occipital lobe epilepsy: 12-years on. J Neurol. 2021 Oct;268(10):3926-3934. https://doi.org/10.1007/s00415-021-10557-y
- Chen C, Shih YH, Yen DJ, Lirng JF, Guo YC, Yu HY, Yiu CH. Olfactory auras in patients with temporal lobe epilepsy. Epilepsia. 2003 Feb;44(2):257-60. https://doi.org/10.1046/j.1528-1157.2003.25902.x
- Fuller GN, Guiloff RJ. Migrainous olfactory hallucinations. J Neurol Neurosurg Psychiatry. 1987 Dec;50(12):1688-90. https://doi.org/10.1136/jnnp.50.12.1688
- Stolte B, Holle D, Naegel S, Diener HC, Obermann M. Vestibular migraine. Cephalalgia. 2015 Mar;35(3):262-70. https://doi.org/10.1177/0333102414535113
- Santana MT, Jackowski AP, da Silva HH, Caboclo LO, Centeno RS, Bressan RA, Carrete H Jr, Yacubian EM. Auras and clinical features in temporal lobe epilepsy: a new approach on the basis of voxel-based morphometry. Epilepsy Res. 2010 May;89(2-3):327-38. https://doi.org/10.1016/j.eplepsyres.2010.02.006
- Angus-Leppan H, Guiloff RJ. Familial limb pain and migraine: 8-year follow-up of four generations. Cephalalgia. 2016;36(11):1086‐1093. https://doi.org/10.1177/0333102415620906
- Guiloff RJ, Fruns M. Limb pain in migraine and cluster headache. J Neurol Neurosurg Psychiatry. 1988 Aug;51(8):1022-31. https://doi.org/10.1136/jnnp.51.8.1022
- Young GB, Blume WT. Painful epileptic seizures. Brain. 1983 Sep;106 (Pt 3):537-54. https://doi.org/10.1093/brain/106.3.537
- Asadi-Pooya AA, Asadollahi M, Sperling MR. Ictal pain: occurrence, clinical features, and underlying etiologies. Epilepsy Behav. 2016;61:59-62. https://doi.org/10.1016/j.yebeh.2016.05.006
- Mínguez-Olaondo A, Quintas S, Morollón Sánchez-Mateos N, López-Bravo A, Vila-Pueyo M, Grozeva V, Belvís R, Santos-Lasaosa S, Irimia P. Cutaneous Allodynia in Migraine: A Narrative Review. Front Neurol. 2022 Jan 21;12:831035. https://doi.org/10.3389/fneur.2021.831035
- Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine?. Brain Behav. 2018;8(6):e00950. https://doi.org/10.1002/brb3.950
- Harris L, Angus-Leppan H. Epilepsy: diagnosis, classification & management, Medicine 2020. https://doi.org/10.1016/j.mpmed.2020.05.001
- Mainieri G, Cevoli S, Giannini G, Zummo L, Leta C, Broli M, Ferri L, Santucci M, Posar A, Avoni P, Cortelli P, Tinuper P, Bisulli F. Headache in epilepsy: prevalence and clinical features. J Headache Pain. 2015;16:556. https://doi.org/10.1186/s10194-015-0556-y
- Bigal ME, Lipton RB, Cohen J, Silberstein SD. Epilepsy and migraine. Epilepsy Behav. 2003;4 Suppl 2:S13-24. https://doi.org/10.1016/j.yebeh.2003.07.003
- Lennox WG, Lennox MA. (1960) Epilepsy and related disorders. Little, Brown & Company, Boston.
- Smyth VO, Winter AI. The EEG in migraine. Electroencephalogr Clin Neurophysiol. 1964 Jan-Feb;16:194-202. https://doi.org/10.1016/0013-4694(64)90038-0
- Parisi P, Paolino MC, Raucci U, Della Vecchia N, Belcastro V, Villa MP, Striano P. Ictal epileptic headache: when terminology is not a moot question. Front Neurol. 2019 Jul 23;10:785. https://doi.org/10.3389/fneur.2019.00785
- Schon F, Blau JN. Post-epileptic headache and migraine. J Neurol Neurosurg Psychiatry. 1987 Sep;50(9):1148-52. https://doi.org/10.1136/jnnp.50.9.1148
- Duko B, Ayalew M, Toma A. The epidemiology of headaches among patients with epilepsy: a systematic review and meta-analysis. J Headache Pain. 2020 Jan 10;21(1):3. https://doi.org/10.1186/s10194-020-1074-0
- Guiloff, RJ (Ed): Clinical trials in Neurology 2001. Springer Verlag, London. https://doi.org/10.1007/978-1-4471-3787-0
- Jackson JL, Cogbill E, Santana-Davila R, Eldredge C, Collier W, Gradall A, Sehgal N, Kuester J. A Comparative Effectiveness Meta-Analysis of Drugs for the Prophylaxis of Migraine Headache. PLoS One. 2015 Jul 14;10(7):e0130733. https://doi.org/10.1371/journal.pone.0130733
- Huang Y, Xiao H, Qin X, Nong Y, Zou D, Wu Y. The genetic relationship between epilepsy and hemiplegic migraine. Neuropsychiatr Dis Treat. 2017;13:1175-1179. Published 2017 Apr 24. https://doi.org/10.2147/NDT.S132451
- Angus-Leppan H, Olausson B, Boers P, Lambert GA. Convergence of afferents from superior sagittal sinus and tooth pulp on cells in the upper cervical spinal cord of the cat. Neurosci Lett. 1994 Dec 5;182(2):275-8. https://doi.org/10.1016/0304-3940(94)90815-X
- Angus-Leppan H, Olausson B, Boers P, Lambert GA. Convergence of afferents from superior sagittal sinus and tooth pulp on cells in the thalamus of the cat. Cephalalgia. 1995 Jun;15(3):191-9. https://doi.org/10.1046/j.1468-2982.1995.015003191.x
