Abstract
Transcranial MR guided Focused ultrasound (MRgFUS) is a recently approved treatment for patients with Essential Tremor (ET), the commonest movement disorder in clinical practice. In this review, we explain why thalamotomy has returned, how it is performed, and outline the basic eligibility criteria and risks of this procedure. The aim of this article is to provide a practical guide to clinicians seeing ET patients as to what they should consider before referring for this treatment.
In November 2020, NHS England published its commissioning document treatment of 150 Essential Tremor (ET) patients a year with Transcranial MR guided Focused ultrasound (MRgFUS). This was remarkable, not only for its timing – its appraisal of the evidence and eligibility criteria were produced at the height of a global pandemic – but also for its endorsement of a treatment by the largest public provider of health care in world, that had hitherto been reserved to a small number of private institutions in North America and Continental Europe. Some might consider this decision to be the product of commercial and patient pressure. In this update, however, we will argue that this was the correct decision, for patients, clinicians and ultimately for the understanding the longer-term role of minimally invasive forms of neurosurgery for neurological symptom control.
Why the return of lesioning?
The therapeutic effect of MRgFUS is achieved by performing a thalamotomy. In this respect, there is nothing “new” about this treatment. Lesioning the thalamus for the relief of tremor is nearly as old as functional neurosurgery itself. Furthermore, ultrasound brain lesions were attempted and quickly abandoned in the 1950s [1].
For many, reports of MRgFUS thalamotomy begged the obvious question of, why? The idea of a return to lesioning seems regressive – were we repeating the mistakes of past generations rather than learning from their experience? Surely thalamotomy went “out with the Ark,” and rightly so, given the unacceptable levels of permanent adverse effects when compared head-to-head with the “reversibility” of Deep Brain Stimulation (DBS) [2]. Like many changes in clinical practice, the reasons are rarely singular and are both obvious and at the same time harder to define. High re-implantation rates [3], the significant cost savings of MRgFUS [4], and patient or clinician antipathy towards open brain surgery might, in part, explain a demand for alternatives to DBS [5, 6].
Technological fusion of Magnetic Resonance Imaging with an ultrasound transducer system that can achieve sub-millimetre resolution thermal ablation is a good starting point. However, these factors do not explain the uptake of MRgFUS over and above more established lesion-based techniques such as radiofrequency ablation or gamma-knife radiosurgery [7]. The principle reasons are two-fold. For the patient, the clinical effects are immediate. There is no period of post-operative uncertainty awaiting the effects of radio-necrosis or months of follow up appointments optimising DBS settings. MRgFUS relies upon delivering low intensity “sonications” (ultrasound doses lasting 10-20 seconds), at intensities that are sub-lesional with the aim of “mapping” the final intended target of the lesion.
During DBS implantation, the number of changes to the electrode trajectory is limited by oedema caused by the electrode tract. In contrast, ultrasound sonications at sub-lesional temperatures can be delivered with a greater spatial freedom to define the final lesion location. This is critical to the success of the procedure as the “eyes” of the surgeon are exclusively guided by feedback from clinical assessment and what the patient experiences. As no meaningful structural imaging can be recovered during the procedure, the heavily clinician led treatment becomes exclusively dependent upon the clinical skills of the neurologist and their communication with the treating surgeon.
Who should be considered for MRgFUS Thalamotomy?
The existing evidence for MRgFUS is supportive of its use in patients with a diagnosis of Essential Tremor targeting the Ventral intermediate nucleus (Vim) of the thalamus[8]. Patients need to be able to tolerate a 2-3 hour period in and out of an MRI scanner making claustrophobia or permanent MR incompatible implants an absolute contraindication. The NHS England eligibility criteria are summarised in Table 1.
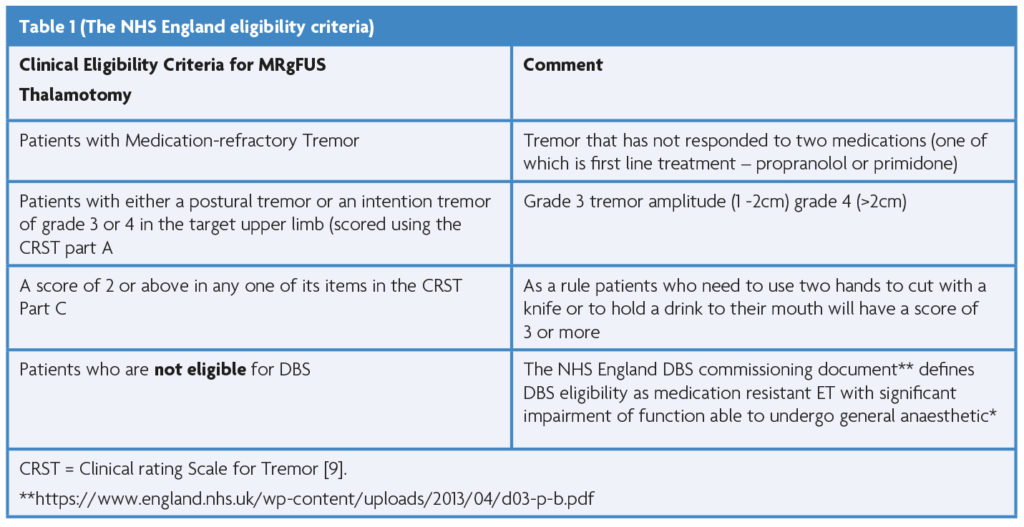
The most challenging aspect of applying these criteria is defining who is “not eligible for DBS.” It is a straightforward decision to offer treatment to a patient over the age of 75 or one with a co-morbidity that general anaesthesia high risk. However, is a 55-year old who is unwilling to consent to DBS given full knowledge of the risk of intracranial surgery ineligible for DBS? This uncertainty places shared, fully informed decision making at the heart of the patient selection process for MRgFUS thalamotomy. It also emphasises the need for a multi-disciplinary approach to ensure that the right patient, despite understandable preference for the “less invasive” option, is offered the correct treatment to ensure the best likelihood of an enduring and robust improvement in their quality of life.
What are the drawbacks of MRgFUS thalamotomy?
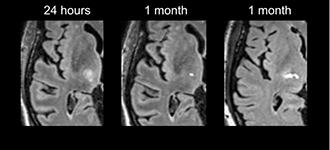
A frequent misperception is that MRgFUS as non-invasive. Relative to open surgery this is true, however, intra-cranial oedema (Figure 1) frequently leads to transient balance and or sensory side effects. Persistence of these can be attributed to lesion extension outside of the target zone [10]. In the pivotal trial of Elias et al., [8] these occurred in over 30% of patients (objective or subjective gait disturbance 36%; paraesthesia/numbness; 38%) persisting at 12 months in around 10% (gait 9%, sensory 14%). These relatively high rates have been replicated in the largest published series [11] with persistent dysarthria observed in 6%. Improved understanding of the relationship between the ultrasound dose and lesion size [11], advances in targeting [12] and intraprocedural imaging, are likely to lead to further reductions in adverse effect profile. However, there remains uncertainty as to what factors explain unpredictable “hyper-response” [7, 11, 13] which occurs in 7% of treatments.
These are associated with “lesion tails” which extend into the internal capsule associated with high level of permanent adverse effects [11]. Accordingly, excluding patients with pre-existing balance and/or gait disorder is considered best practice and is reflected in existing guidelines. This can mean that many patients who have severe ET are excluded based upon gait abnormalities which are a common “soft” sign in ET [14]. The risk of permanent gait disturbance also needs to be considered in younger ET patients who are unwilling to consent to the risks of DBS. A handful of patients have gone on to have DBS following MRgFUS [15, 16], however, in the worst-case scenario, permanent side effects from a MRgFUS treatment may eliminate DBS as a follow on “rescue” therapy.
MRgFUS exclusively aims at improving (typically dominant) limb tremor. Head and voice tremor do not respond to unilateral treatment [8] so patients with more axial tremor symptom burden may be more appropriate for DBS. Historical concerns about risk of dysarthria from traditional thalamotomy have led to considerable caution when performing bilateral MRgFUS thalamotomy[2]. Results from a recently published trial where bilateral ultrasound thalamotomy was performed at least year after the first hemisphere was treated, look promising from a safety point of view [17]. However, experience is so limited that it should not be considered outside of a research context.
Most patients receive a significant improvement in tremor control in the treated limb. In around 10% [18] the tremor returns to baseline levels and one third see less than 50% improvement at two-year follow up [19]. Re-treatment is possible but is technically more challenging at the second attempt at thalamotomy. It is important therefore, for patient expectations to be adequately managed pre-treatment in the event of treatment failure.
What is the future likely to hold for MRgFUS?
One of the biggest outstanding questions is whether MRgFUS has a role in the treatment of non-ET tremor syndromes. Tremor dominant Parkinson’s disease (PD-T) being the most obvious indication. The existing evidence is limited in quantity and quality to support its use in PD-T outside of clinical research [20, 21]. To date, outcomes from MRgFUS thalamotomies in PD-T show similar safety profiles to ET studies but much greater treatment variability. Notably, no long-term follow-up data is available to inform whether the clinical effect is durable. Whether these limitations of MRgFUS in PD-T reflect difficulties in selecting patients from a heterogeneous disease group (cf. ET), or uncertainty as to the most efficacious target for tremor, are questions subject to ongoing research investigations [22]. It seems likely, with the appropriately rigorously designed clinical studies, these questions will be more clearly answered and a new treatment option available to other patient groups who experience life limiting tremor.
References
- Fry WJ, et al. Production of focal destructive lesions in the central nervous system with ultrasound. Journal of neurosurgery, 1954. 11(5): p. 471-478. https://doi.org/10.3171/jns.1954.11.5.0471
- Schuurman PR, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. New England Journal of Medicine. 2000; 342(7):461-468. https://doi.org/10.1056/NEJM200002173420703
- Rolston JD, et al. An unexpectedly high rate of revisions and removals in deep brain stimulation surgery: analysis of multiple databases. Parkinsonism & related disorders. 2016;33:72-77. https://doi.org/10.1016/j.parkreldis.2016.09.014
- Jameel A, et al. The cost-effectiveness of unilateral magnetic resonance-guided focused ultrasound in comparison with unilateral deep brain stimulation for the treatment of medically refractory essential tremor in England. The British Journal of Radiology. 2022;95(1140):202. https://doi.org/10.1259/bjr.20220137
- Hamberg K, Hariz G-M. The decision-making process leading to deep brain stimulation in men and women with parkinson’s disease-an interview study. BMC neurology. 2014;14(1):1-10. https://doi.org/10.1186/1471-2377-14-89
- Das S, et al. Capturing Initial Understanding and Impressions of Surgical Therapy for Parkinson’s Disease. Frontiers in neurology, 2021;12:214. https://doi.org/10.3389/fneur.2021.605959
- Iorio-Morin C, Hodaie M, Lozano AM. Adoption of focused ultrasound thalamotomy for essential tremor: why so much fuss about FUS? Journal of Neurology, Neurosurgery & Psychiatry. 2021;92(5):549-554. https://doi.org/10.1136/jnnp-2020-324061
- Elias WJ, et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med. 2016;375(8):730-9. https://doi.org/10.1056/NEJMoa1600159
- Fahn S, Tolosa E, Marín C. Clinical rating scale for tremor. Parkinson’s disease and movement disorders. 1993;2:271-280.
- Boutet A, et al. Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain. 2018;141(12):3405-3414. https://doi.org/10.1093/brain/awy278
- Segar DJ, et al. Lesion location and lesion creation affect outcomes after focused ultrasound thalamotomy. Brain. 2021;144(10):3089-3100. https://doi.org/10.1093/brain/awab176
- Krishna V, et al. Prospective tractography-based targeting for improved safety of focused ultrasound thalamotomy. Neurosurgery. 2019;84(1):160-168. https://doi.org/10.1093/neuros/nyy020
- Rohani M, Fasano A. Focused ultrasound for essential tremor: review of the evidence and discussion of current hurdles. Tremor and Other Hyperkinetic Movements, 2017;7. https://doi.org/10.5334/tohm.378
- Stolze H, et al. The gait disorder of advanced essential tremor. Brain. 2001;124(11):2278-2286. https://doi.org/10.1093/brain/124.11.2278
- Wang TR, et al. Thalamic deep brain stimulation salvages failed focused ultrasound thalamotomy for essential tremor: a case report. Stereotactic and Functional Neurosurgery. 2018;96(1):60-64. https://doi.org/10.1159/000486646
- Saluja S, et al. Case report on deep brain stimulation rescue after suboptimal MR-guided focused ultrasound thalamotomy for essential tremor: a tractography-based investigation. Frontiers in human neuroscience, 2020:191. https://doi.org/10.3389/fnhum.2020.00191
- Iorio-Morin C, et al. Bilateral Focused Ultrasound Thalamotomy for Essential Tremor (BEST-FUS Phase 2 Trial). Mov Disord. 2021. https://doi.org/10.1002/mds.28716
- Sinai A, et al. Magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: a 5-year single-center experience. Journal of Neurosurgery. 2019; 133(2):417-424. https://doi.org/10.3171/2019.3.JNS19466
- Chang JW, et al. A prospective trial of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: Results at the 2‐year follow‐up. Annals of neurology. 2018;83(1):107-114. https://doi.org/10.1002/ana.25126
- Schlesinger I, et al. MRI guided focused ultrasound thalamotomy for moderate-to-severe tremor in Parkinson’s disease. Parkinson’s disease. 2015. https://doi.org/10.1155/2015/219149
- Bond AE, et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: a randomized clinical trial. JAMA neurology, 2017;74(12):1412-1418. https://doi.org/10.1001/jamaneurol.2017.3098
- Jameel A, et al. Double lesion MRgFUS treatment of essential tremor targeting the thalamus and posterior sub-thalamic area: preliminary study with two year follow-up. British Journal of Neurosurgery. 2022;36(2):241-250. https://doi.org/10.1080/02688697.2021.1958150
