Abstract
Multiple System Atrophy (MSA) is a fatal neurodegenerative disease with a mean survival of 10 years after symptom onset. The pathological characteristics of MSA are glial cytoplasmic inclusions (GCIs) in oligodendrocytes. There is an urgent need to further understand the pathophysiological mechanisms involved in MSA, and to find disease modifying treatments which slow disease progression. Pre-clinical research has suggested the presence of insulin resistance in the MSA brain and that a type 2 diabetes mellitus (T2DM) drug, exenatide, has the potential to be a disease modifying treatment for MSA. In this review, I discuss the pre-clinical evidence for this approach in MSA as well as propose possible outcome measures for use in any such MSA clinical trial.
Multiple System Atrophy (MSA) is categorised as an atypical parkinsonian syndrome. It is a rare disease with an approximate prevalence of 5 per 100,000 people worldwide.1 Patients with MSA have an estimated median survival of 10 years after symptom onset.2,3 The clinical features of MSA include autonomic dysfunction, parkinsonism and cerebellar ataxia. There are two main types of MSA which are classified depending on the patients predominant motor features; MSA-P is a type of MSA where patients present with clinical signs of parkinsonism such as tremor and rigidity, and MSA-C is the MSA type where patients predominantly show features of cerebellar impairment for example, dysarthria and ataxia.4 The pathological hallmark of MSA is glial cytoplasmic inclusions (GCIs). These protein aggregates, which have been found in the brain oligodendrocytes, contain alpha-synuclein, hence MSA is additionally known as an alpha-synucleinopathy. GCIs have typically been found located in the areas where neurodegeneration most occurs in the MSA brain; namely the striatonigral and olivopontocerebellar systems.5
MSA is a rapidly progressive disease that currently has no disease modifying treatment. Recent research into the aetiology and pathophysiological mechanisms of MSA has suggested the presence of insulin resistance. A drug licenced for treating type 2 diabetes mellitus (T2DM), exenatide, has therefore been proposed to be a possible treatment option in slowing down the progression of MSA. Exenatide (synthetic version exendin-4) is a glucagon-like peptide-1 (GLP-1) receptor agonist which is, in normal physiological conditions, activated by the GLP-1 hormone secreted by L-cells in the gut after ingestion of food; to induce insulin secretion, reduce glucagon secretion and to reduce appetite. The mechanisms of action of exenatide in T2DM is similar to GLP-1 in that it reduces hyperglycaemia, induces a glucose-dependent increase of insulin secretion and decrease of abnormally elevated glucagon secretion, slows gastric emptying and lowers food intake.7
Insulin-like growth factor-1 (IGF-1) is a hormone predominantly secreted by the liver but can also be produced by many other organs including the brain. It has a broad range of functions within the central nervous system which include modulating early brain development, oligodendrogenesis and myelination as well a role in synaptic neurotransmission. Moreover, IGF-1 may also play a role in neuroinflammation.8 An abnormally high level of IGF-1 as measured in bodily fluids (blood or cerebrospinal fluid) would suggest the existence of a problem in the IGF-1 and/or insulin signalling system in the body possibly pointing towards the presence of insulin resistance. Exenatide activates the same effectors as IGF-1 and therefore, has the potential to activate or deactivate pathways associated with IGF-1 if there is any dysfunction.
A study investigating biomarkers in MSA has shown that there are significantly increased serum levels of IGF-1 in MSA (n = 25) compared to healthy controls (n = 25).9 This finding was validated in a second study that showed a significant elevation in levels of serum IGF-1 in MSA (n = 25) when compared to healthy controls (n = 52) and those with Parkinson’s disease (PD, n = 79).10 Additionally, IGF-1 levels positively correlated with MSA disease duration and the MSA motor function assessing scale, the United Multiple System Atrophy Rating Scale (UMSARS) part II.10 These results imply there is an abnormality in the processing of IGF-1 in the body, possibly insulin resistance, which is leading to higher serum levels of IGF-1 in MSA. The results also suggest that serum IGF-1 levels correlate with disease severity in MSA, although no causal links have yet been found between these two findings.
Further investigation into insulin resistance in MSA using mouse models and human brain samples has also supported the concept of insulin resistance in the MSA brain. Immunofluorescent staining techniques using MSA brain samples (n = 7, controls n = 5) found that neurones in the putamen expressed higher levels of insulin resistance markers (IRS-1pS312 and IRS-1pS616). Furthermore, the levels of the IRS-1pS312 marker correlated positively with disease duration, again pointing towards an association between the degree of insulin resistance in the brain and disease severity. Investigation into MSA patient oligodendrocytes also showed the presence of the insulin resistant markers however, specifically IRS-1pS312 expression was found to be most apparent in the oligodendrocytes that contained GCIs in MSA.11
Insulin resistance in MSA has been further explored in the PLP-SYN transgenic mice; a model expressing human wild-type alpha-synuclein using a oligodendrocyte specific proteolipid (PLP) promotor.11,12 In this model, both GCI-like structures develop in oligodendrocytes and clinical features of MSA, such as bladder dysfunction13 and parkinsonism14. Investigation into insulin resistance in the PLP-SYN model (n = 7, wildtype n = 7) further supported the above finding of insulin resistance in the MSA brain because the PLP-SYN mice had a significantly higher level of insulin marker IRS-1pS307, equal to the human IRS-1pS312, in the striatum compared to the wildtype controls. Furthermore, in this study exenatide was given to the PLP-SYN mice to see whether it had an effect on insulin resistance, cell death and disease progression namely, any disease modifying effect on MSA. 9 six-week year old PLP-SYN mice were given placebo, 9 were given 3.5 pmol/kg/min of exendin-4 and 7 mice were given a higher dose of 8.75 pmol/kg/min of exendin-4 for twelve weeks. Analyses of the three groups showed that the administration of exendin-4 reduced insulin resistance in the brain and decreased cell death. In addition, the higher dose of exendin-4 significantly reduced alpha-synuclein load in the striatum although, disappointingly, behaviour and motor symptom analyses did not show any significant improvement in motor performance with administration of exendin-4.11
Overall, these results suggest that exenatide does have potential in slowing down disease progression in MSA, but does it have the potential to reduce motor progression in humans? This is a question only a clinical trial can answer.
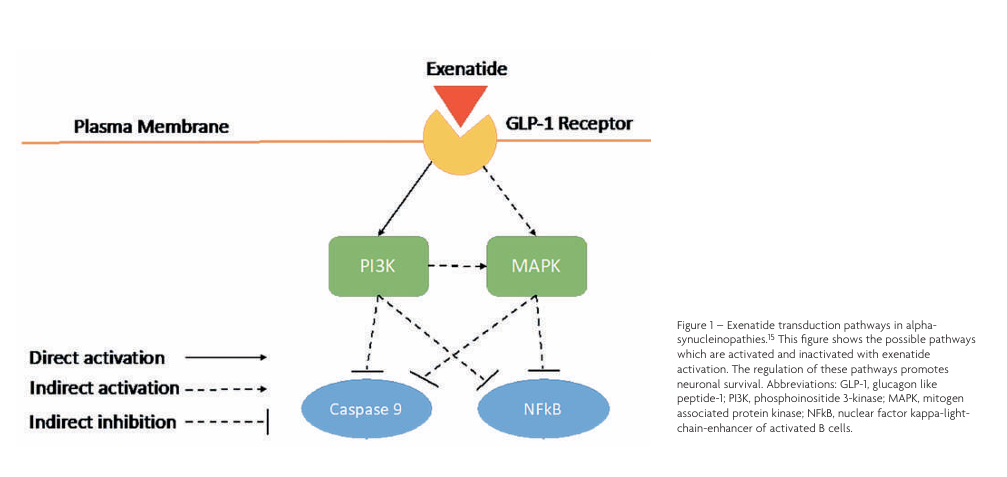
The exact mechanism by which exenatide promotes neuronal survival is not entirely understood, however, knowledge of pathways associated with GLP-1 receptor activation (as seen in Figure 1) demonstrates that in neurones, exenatide activates the phosphoinositide 3-kinase (PI3K) pathway which promotes axonal growth, neuronal regeneration and protein synthesis. Additionally, the receptor activates the mitogen associated protein kinase (MAPK) pathway which inhibits apoptosis and encourages neuronal survival. Both activation of PI3K and MAPK pathways inhibit caspase 9, blocking apoptosis. Consequently, these pathways also inhibit the nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) pathway preventing oxidative stress and neuroinflammation.15
Exenatide has been studied previously as a disease modifying drug for Parkinson’s disease (PD) in a single-centre, randomised, double-blind, placebo-controlled trial in 62 patients with PD; 32 patients treated with exenatide and 30 patients treated with placebo. Patients self-administered exenatide (2 mg) or placebo subcutaneously once weekly for 48 weeks. They were followed up every 12 weeks from baseline for a total of 60 weeks. The results of the trial showed that the mean change in Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) motor subscale (part 3) scores in the exenatide group were significantly different to the placebo group with the exenatide group having an improvement in motor scores over time, in contrast to the placebo group .16 This result could be due to the drug truly having a disease modifying effect, however it may be because the participants in the exenatide group plateaued in their disease progression and the therapy is only having a symptomatic effect. Further research needs to be conducted with a larger cohort in order to validate these results and this is currently ongoing in an about to start Phase III study.
The exenatide in PD trial illustrates that to conduct a successful clinical trial for MSA, there must be a reliable means of showing a potential treatment effect. Currently, the main way to measure disease modification in MSA is by using UMSARS scores. UMSARS scores take into account the ability of the patient to conduct their activities of daily living (UMSARS I-Historical Review section), their movement features (UMSARS-II Motor Examination), their autonomic problems (UMSARS-III Autonomic Examination) and their Global Disability Scale score (UMSARS-IV). Similar to MDS-UPDRS, UMSARS scores are a subjective measure, and this suggests that there may be bias and potential inter-rater variability in the measurements. Hence, UMSARS may not be the ideal way to demonstrate any disease modifying effect of exenatide.
An objective biomarker of treatment efficacy would be a better way of monitoring disease modification. Unfortunately, there is no such biomarker for MSA although recent research into neurofilament light (NfL) levels has shown promising results. NfL is a biomarker of axonal damage and CSF and serum levels have been shown to correlate with disease progression in many neurodegenerative diseases including MSA.21,22 Moreover, as mentioned previously, IGF-1 levels in the serum of patients with MSA was found to correlate with disease severity thus, IGF-1 levels may also have the potential to objectively correspond to disease modification in a clinical investigation.10 Combining IGF-1 and NfL levels in bodily fluids with UMSARS scores has the potential to be a better more objective endpoint in assessing disease modification and progression in a clinical trial for MSA.
Other disease modifying treatments that are being investigated for MSA through non-clinical or clinical trials include mesenchymal stem cell therapy17, Verdiperstat (a myeloperoxidase inhibitor)18, CoQ10 supplementation19 and new molecules aiming to block oligomeric alpha-synuclein from forming new toxic oligomers20.
In summary, pre-clinical experiments have revealed insulin resistance in the MSA brain. Additionally, these experiments suggest that exenatide, a T2DM drug, has the potential to function as a disease modifying treatment for MSA through this pathway. More research is required to find more reliable biomarkers which correlate with disease severity and progression to inform MSA clinical trial design and help assess whether exenatide truly has disease modifying effects in MSA.
References
- Levin J, Kurz A, Arzberger T, Giese A, Höglinger GU. The Differential Diagnosis and Treatment of Atypical Parkinsonism. Dtsch Arztebl Int. 2016;113(5):61‐69. https://doi.org/10.3238/arztebl.2016.0061
- Wenning GK, Geser F, Krismer F, et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 2013;12(3):264‐274.
- Low PA, Reich SG, Jankovic J, et al. Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol. 2015;14(7):710‐719. https://doi.org/10.1016/S1474-4422(15)00058-7
- Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670‐676. https://doi.org/10.1212/01.wnl.0000324625.00404.15
- Tu PH, Galvin JE, Baba M, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44(3):415‐422. https://doi.org/10.1002/ana.410440324
- int. 2020. Diabetes. [online] Available at: https://www.who.int/news-room/fact-sheets/detail/diabetes [Accessed 14 May 2020].
- Bhavsar S, Mudaliar S, Cherrington A. Evolution of exenatide as a diabetes therapeutic. Curr Diabetes Rev. 2013;9(2):161‐193. https://doi.org/10.2174/157339913805076472
- Labandeira-Garcia JL, Costa-Besada MA, Labandeira CM, Villar-Cheda B, Rodríguez-Perez AI. Insulin-Like Growth Factor-1 and Neuroinflammation. Front Aging Neurosci. 2017;9:365. Published 2017 Nov 3. https://doi.org/10.3389/fnagi.2017.00365
- Pellecchia MT, Pivonello R, Longo K, et al. Multiple system atrophy is associated with changes in peripheral insulin-like growth factor system. Mov Disord. 2010;25(15):2621‐2626. https://doi.org/10.1002/mds.23320
- Numao A, Suzuki K, Miyamoto M, Miyamoto T, Hirata K. Clinical correlates of serum insulin-like growth factor-1 in patients with Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. Parkinsonism Relat Disord. 2014;20(2):212‐216. https://doi.org/10.1016/j.parkreldis.2013.11.005
- Bassil F, Canron MH, Vital A, et al. Insulin resistance and exendin-4 treatment for multiple system atrophy. Brain. 2017;140(5):1420‐1436. https://doi.org/10.1093/brain/awx044
- Kahle PJ, Neumann M, Ozmen L, et al. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3(6):583‐588. https://doi.org/10.1093/embo-reports/kvf109
- Boudes M, Uvin P, Pinto S, et al. Bladder dysfunction in a transgenic mouse model of multiple system atrophy. Mov Disord. 2013;28(3):347‐355. https://doi.org/10.1002/mds.25336
- Refolo V, Bez F, Polissidis A, et al. Progressive striatonigral degeneration in a transgenic mouse model of multiple system atrophy: translational implications for interventional therapies. Acta Neuropathol Commun. 2018;6(1):2. https://doi.org/10.1186/s40478-017-0504-y
- Bassil F, Fernagut PO, Bezard E, Meissner WG. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification? Prog Neurobiol. 2014;118:1‐18. https://doi.org/10.1016/j.pneurobio.2014.02.005
- Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1664‐1675. doi:10.1016/S0140-6736(17)31585-4 https://doi.org/10.1016/S0140-6736(17)31585-4
- Singer W, Dietz AB, Zeller AD, et al. Intrathecal administration of autologous mesenchymal stem cells in multiple system atrophy. Neurology. 2019;93(1):e77‐e87. https://doi.org/10.1212/WNL.0000000000007720
- Stefanova N, Georgievska B, Eriksson H, Poewe W, Wenning GK. Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox Res. 2012;21(4):393‐404. https://doi.org/10.1007/s12640-011-9294-3
- Mitsui J, Koguchi K, Momose T, et al. Three-Year Follow-Up of High-Dose Ubiquinol Supplementation in a Case of Familial Multiple System Atrophy with Compound Heterozygous COQ2 Mutations. Cerebellum. 2017;16(3):664‐672. https://doi.org/10.1007/s12311-017-0846-9
- gov. 2020. A First-In-Human Study Of Single And Multiple Doses Of Anle138b In Healthy Subjects – Full Text View – Clinicaltrials.Gov. [online] Available at: https://www.clinicaltrials.gov/ct2/show/NCT04208152 [Accessed 23 May 2020].
- Hansson, O., Janelidze, S., Hall, S., Magdalinou, N., Lees, A., Andreasson, U., Norgren, N., Linder, J., Forsgren, L., Constantinescu, R., Zetterberg, H. and Blennow, K. (2017). Blood-based NfL: A Biomarker for Differential Diagnosis of Parkinsonian Disorder. Neurology, 88(10), pp.930-937. https://doi.org/10.1212/WNL.0000000000003680
- Magdalinou, N., Paterson, R., Schott, J., Fox, N., Mummery, C., Blennow, K., Bhatia, K., Morris, H., Giunti, P., Warner, T., de Silva, R., Lees, A. and Zetterberg, H. (2015). A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. Journal of Neurology, Neurosurgery & Psychiatry, 86(11), pp.1240-1247. https://doi.org/10.1136/jnnp-2014-309562
