Abstract
Necrotising Autoimmune Myopathy is a subacute proximal myopathy with high creatine kinase levels and biopsy findings of necrotic and regenerating fibres with minimal inflammation. It is associated with anti-SRP and anti-HMGCR antibodies, malignancy and connective tissue disorders, and is responsive to immunotherapy. This review aims to increase clinician awareness of this rare but potentially treatable condition, by describing the clinical presentation, serological and biopsy findings, and providing an overview of the currently utilised immunotherapy regimens.
Key points
- NAM presents with subacute proximal muscle weakness and very high serum creatine kinase levels
- It is associated with anti-HMGCR and anti-SRP antibodies, connective tissue disease and malignancy
- It is responsive to immunotherapy, often requiring multiple immunosuppressive agents
- Rituximab and IVIG are being increasingly used in severe and refractory disease
Introduction
Necrotising Autoimmune Myopathy (NAM) is a relatively newly recognised subtype of the immune-mediated myopathies, characterised clinically by the subacute onset of proximal muscle weakness, often with a significantly raised creatine kinase (CK) level. It is associated with 3-hydroxy-3-methylglutaryl–CoA reductase (anti-HMGCR) antibody (with or without statin medication exposure), Signal Recognition Particle (anti-SRP) antibody, connective tissue disorders, and malignancy.1-3 In addition, there have been case reports of NAM associated with hepatitis C and HIV.1 Electromyography shows increased insertional and spontaneous activity, 4 and muscle biopsy reveals necrotic and regenerating fibres, with minimal inflammation.4 Although there are no prospective trials, treatment generally involves immunotherapy, with the majority requiring multiple immunotherapy agents, with high rates of relapse 5–7 (see Table 1).
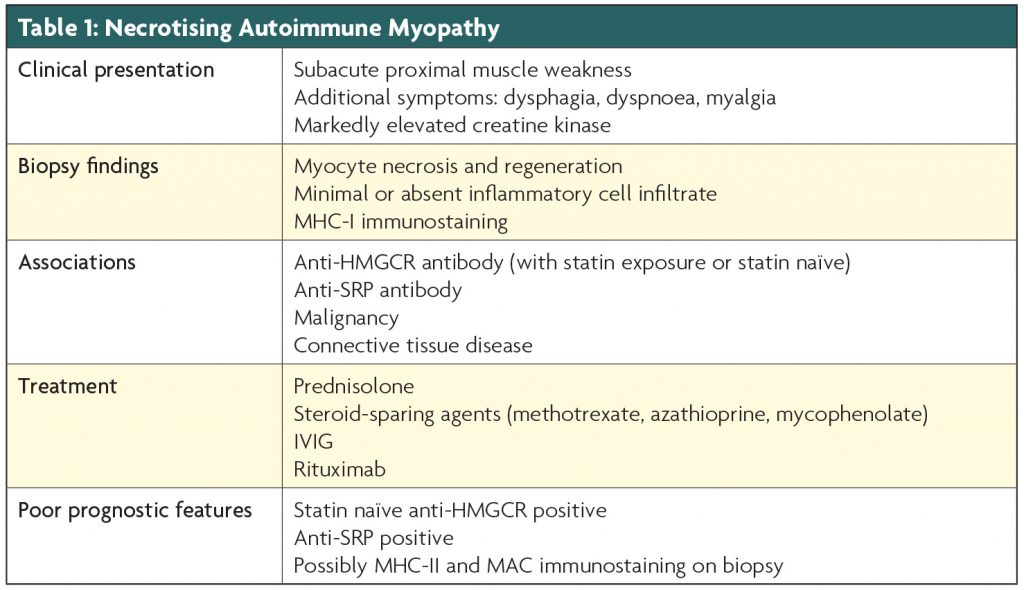
Clinical presentation
NAM presents sub-acutely with symptoms of hip and shoulder-girdle muscle weakness, such as difficulty rising from low chairs, climbing stairs or lifting weights above the head, and is clinically very similar to polymyositis,8 although patients often appear to have more muscle atrophy on presentation.1,9 The clinical criteria for the diagnosis of NAM, from the 119th ENMC International Workshop on Idiopathic Inflammatory Myopathies, requires a subacute or insidious onset of proximal muscle weakness, with neck flexor rather than extensor weakness, associated with an elevated serum CK level, and no ocular weakness.4 NAM can also be associated with dysphagia, dyspnoea and myalgia,1,3,5,9 and there have been reports of cardiac involvement and interstitial lung disease, especially in anti-SRP positive patients.1,5,9 It is important to exclude toxic myopathies, thyroid disease and muscular dystrophies, as these conditions can have similar biopsy findings. CK levels are generally very high, often more than ten times the upper limit of normal.3,10 Electromyography is consistent with an inflammatory myopathy, showing increased insertional activity, fibrillation potentials, positive sharp waves or complex repetitive discharges, as well as short, small amplitude, polyphasic motor unit potentials.4
Aetiological associations
All patients with suspected NAM should have serology performed for anti-HMGCR, anti-SRP and myositis specific antibodies, as well as ANA and ENAs, and blood-borne viruses including HIV and Hepatitis.
HMGCR antibodies
Christopher-Stine et al first discovered the presence of an autoantibody in a subset of statin-exposed patients with NAM,11 which was subsequently identified as the HMGCR autoantibody by Mammen et al.12 The commercially available anti-HMGCR ELISA has a sensitivity of 94.4% and specificity of 99.3%, and to date there have not been any published cases of a positive anti-HMGCR antibody without associated muscle disease.13 A new diagnostic algorithm, published by Andrew Mammen in 2016, suggests in patients over the age of 65 years with proximal muscle weakness and a high CK level that does not resolve within two months of statin cessation, anti-HMGCR serology should be performed, and if positive, a presumptive diagnosis of NAM may be made.10 This can then be confirmed by muscle biopsy. The anti-HMGCR antibody has also been found in patients without prior statin exposure.13 These statin naïve patients tend to be younger with severe muscle weakness, which is more refractory to immunotherapy than the statin-exposed patients.14 In the statin exposed patients, the development of NAM is not always temporally associated with statin commencement,15often starting years after first exposure, so proving causality is difficult. Indeed some have questioned whether statins play a role, however most case series agree that when these generally older statin-exposed patients develop this disease, it is milder and easier to treat than the younger statin-naïve cohort.
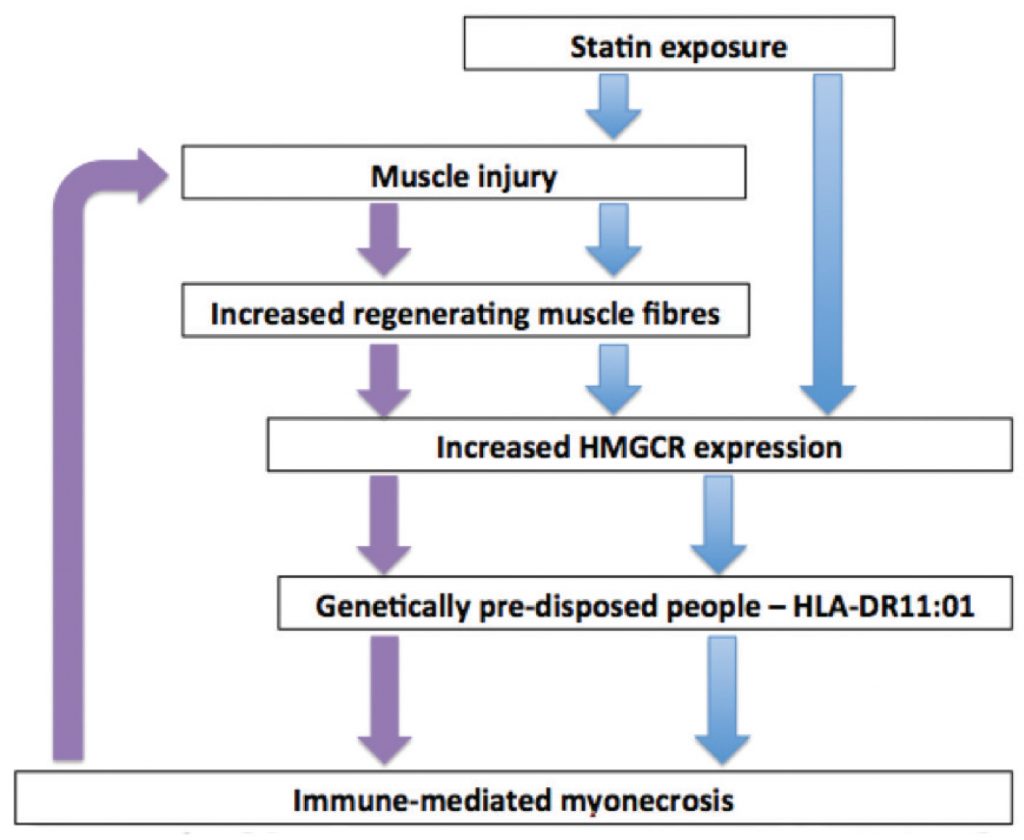
Anti-HMGCR associated NAM has been strongly associated with HLA-DRB11*01(16,17), confirmed again in a recent Japanese series,18 who also reported an association between HLA-DRB1*0803 and statin-associated NAM. The proposed pathogenic pathway is shown in Figure 1: statin exposure upregulates HMGCR expression in muscle cells via both the drug’s direct effect and via muscle injury with resultant muscle fibre regeneration, as HMGCR expression is increased in regenerating muscle fibres compared with resting myocytes. In genetically predisposed individuals, (those that have HLA-DRB1*1101) it is postulated that the presentation of HMGCR-derived peptides to the immune system is a possible pathogenic pathway leading to autoimmunity against HMGCR, which is then sustained in a vicious cycle, months to years after statin cessation, due to the ongoing HMGCR expression in the regenerating myocytes, as suggested by Mammen et al.10,18,19
SRP antibodies
Antibodies against the Signal Recognition Particle are not specific to NAM and have been found in patients with systemic sclerosis and anti-synthetase syndrome.20, 21 Patients with NAM and positive anti-SRP antibodies tend to have a rapidly progressive disease course with severe weakness and disability,1 although there have been case reports of a more insidious disease course that can mimic muscular dystrophy.9 Interstitial lung disease and cardiac involvement are most frequently reported in NAM patients with anti-SRP antibodies.1 Patients with anti-SRP associated NAM tend to have more refractory disease and are less responsive to single agent immunotherapy.9 Ohnuki et al found a significant association between HLA DRB1*0803 and anti-SRP associated NAM in their Japanese cohort.18
Connective tissue disease
Rheumatological conditions can present with necrotising myopathy in an overlap syndrome.21 There have been case reports of NAM in association with Sjogren’s disease, Scleroderma, and Systemic Lupus Erythematosus,3,22 although connective tissue disease associated NAM is thought to be less common than anti-HMGCR and anti-SRP antibody associated NAM.3,5
Malignancy
According to the literature, approximately 10% of all NAM cases are paraneoplastic.5,23 While there are insufficient patient numbers to specify which cancers are most culpable, there have been numerous reports of NAM associated with gastrointestinal, breast and lung cancers.3,24,25 A large cohort study (Allenbach et al, 2016) found a significantly increased incidence of cancer within three years of diagnosis in patients with anti-HMGCR antibodies, as well as NAM patients with no myositis specific antibodies, and on the basis of these results, recommended formal malignancy screening in patients over 50 years with these serological results.25 Anti-SRP associated NAM does not appear to be associated with malignancy.25
Biopsy
Muscle biopsy, usually of the vastus lateralis or deltoid, should be performed prior to immunotherapy commencement to facilitate accurate diagnosis. Typically, NAM histologically shows a pauci-immune necrotizing myositis, characterised by necrotic and regenerating fibres with minimal or absent inflammatory cell infiltrate, with CD163+ macrophages being the most prominent cell type.3,26 The ENMC criteria specify that only sparse perivascular inflammation may be present, with perimysial inflammation excluded.4 Further studies have found an association with MHC-1 sarcolemmal deposition.26,27 MAC sarcolemmal deposition is also increasingly recognised,3,27 particularly in cases associated with anti-SRP antibodies,28 and may be a marker of more severe disease.29
Treatment
There are no randomised controlled trials to direct management, and therefore we are guided by case series and expert opinion. Where there is an obvious underlying cause, such as malignancy, this needs to be treated. NAM appears responsive to corticosteroids, IVIG and rituximab, with many patients requiring multiple agents, particularly the statin-naive anti-HMGCR and anti-SRP positive patients.29,30 Treatment is moving towards early aggressive immunotherapy, particularly in these sub-groups. Kassardjian et al found that treatment with two or more immunotherapeutic medications within the first 3 months of onset predicted a more favourable outcome.5
The agents used vary between case series, influenced by individual experience and local financial constraints. Our general approach in Australia is to initiate high dose corticosteroids in combination with a steroid-sparing medication (such as methotrexate, azathioprine or mycophenolate), with dosage adjustments determined by clinical and biochemical response. If at 3 months the response is incomplete, or if the patient is in one of the poorer prognostic groups with a florid clinical presentation, then IVIG and/or rituximab is added. Some patients relapse on steroid weaning, requiring an additional agent (such as IVIG, Rituximab or cyclosporine). Prospective studies are needed to confirm the most effective regimens for the different subtypes.
Conclusion
Increasingly NAM is recognised as one of the most common immune-mediated myopathies. It is associated with specific antibodies in the majority of cases, most commonly anti-HMGCR and anti-SRP. These antibodies form a central part of the subtype diagnosis, predicting clinical course and possible complications. It is an important condition to recognise and distinguish from other forms of myocyte necrosis and regeneration, as it is responsive to immunotherapy.
References
- Basharat P, Christopher-Stine L. Immune-Mediated Necrotizing Myopathy: Update on Diagnosis and Management. Curr Rheumatol Rep [Internet]. 2015 Dec [cited 2016 Feb 14];17(12). Available from: http://link.springer.com/10.1007/s11926-015-0548-6
- Allenbach Y, Benveniste O. Acquired necrotizing myopathies: Curr Opin. 2013;26(5):554–60.
- Liang C, Needham M. Necrotizing autoimmune myopathy: Curr Opin Rheumatol. 2011;23(6):612–9.
- Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, et al. 119th ENMC international workshop: Trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul Disord. 2004;14(5):337–45.
- Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical Features and Treatment Outcomes of Necrotizing Autoimmune Myopathy. JAMA Neurol. 2015 Sep 1;72(9):996.
- Suzuki S, Hayashi YK, Kuwana M, Tsuburaya R, Suzuki N, Nishino I. Myopathy Associated With Antibodies to Signal Recognition Particle: Disease Progression and Neurological Outcome. Arch Neurol [Internet]. 2012 Jun 1 [cited 2016 Feb 14];69(6). Available from: http://archneur.jamanetwork.com/article.aspx?doi=10.1001/archneurol.2011.1728
- Ramanathan S, Langguth D, Hardy TA, Garg N, Bundell C, Rojana-Udomsart A, et al. Clinical course and treatment of anti-HMGCR antibody-associated necrotizing autoimmune myopathy. Neurol Neuroimmunol Neuroinflammation. 2015 Apr 2;2(3):e96–e96.
- Albayda J, Mammen AL. Is Statin-Induced Myositis Part of the Polymyositis Disease Spectrum? Curr Rheumatol Rep. 2014 Jul 5;16(8):1–6.
- Watanabe Y, Uruha A, Suzuki S, Nakahara J, Hamanaka K, Takayama K, et al. Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy. J Neurol Neurosurg Psychiatry. 2016 May 4;jnnp-2016-313166.
- Mammen AL. Statin-Associated Autoimmune Myopathy. N Engl J Med. 2016 Feb 18;374(7):664–9.
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62(9):2757–66.
- Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63(3):713–21.
- Mammen AL, Pak K, Williams EK, Brisson D, Coresh J, Selvin E, et al. Rarity of anti–3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase antibodies in statin users, including those with self‐limited musculoskeletal side effects. Arthritis Care Amp Res. 2012 Feb 1;64(2):269–72.
- Mohassel P, Mammen AL. Statin-associated autoimmune myopathy and anti-HMGCR autoantibodies: Anti-HMGCR-Associated Myopathy. Muscle Nerve. 2013;48(4):477–83.
- Grable‐Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune‐mediated necrotizing myopathy associated with statins. Muscle Amp Nerve. 2010 Feb 1;41(2):185–90.
- Mammen AL, Gaudet D, Brisson D, Christopher-Stine L, Lloyd TE, Leffell MS, et al. Increased frequency of DRB1*11:01 in anti–hydroxymethylglutaryl-coenzyme A reductase–associated autoimmune myopathy. Arthritis Care Res. 2012 Aug 1;64(8):1233–7.
- Limaye V, Bundell C, Hollingsworth P, Rojana-Udomsart A, Mastaglia F, Blumbergs P, et al. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve. 2015 Aug 1;52(2):196–203.
- Ohnuki Y, Suzuki S, Shiina T, Uruha A, Watanabe Y, Suzuki S, et al. HLA-DRB1 alleles in immune-mediated necrotizing myopathy. 2016 Aug 31;10.1212/WNL.0000000000003160.
- Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63(3):713–21.
- Benveniste O, Drouot L, Jouen F, Charuel J-L, Bloch‐Queyrat C, Behin A, et al. Correlation of anti–signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Amp Rheum. 2011 Jul 1;63(7):1961–71.
- Mammen AL. Autoimmune myopathies: autoantibodies, phenotypes and pathogenesis. Nat Rev Neurol. 2011;7(6):343.
- O’Grady J, Harty L, Mayer N, Critcher V, Ryan J. Immune-Mediated Necrotizing Myopathy, Associated With Antibodies to Signal Recognition Particle, Together With Lupus Nephritis: Case Presentation and Management. J Clin Med Res. 2015 Apr 8;7(6):490–4.
- Wegener S, Bremer J, Komminoth P, Jung HH, Weller M. Paraneoplastic Necrotizing Myopathy with a Mild Inflammatory Component: A Case Report and Review of the Literature. Case Rep Oncol. 2010 Apr 8;3(1):88–92.
- Stenzel W, Goebel H-H, Aronica E. Review: Immune-mediated necrotizing myopathies – a heterogeneous group of diseases with specific myopathological features: Immune mediated necrotizing myopathies. Neuropathol Appl Neurobiol. 2012;38(7):632–46.
- Allenbach Y, Keraen J, Bouvier A, Jooste V, Champtiaux N, Hervier B, et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. 2016 Aug 1;139(8):2131–5.
- Chung T, Christopher-Stine L, Paik JJ, Corse A, Mammen AL. The composition of cellular infiltrates in anti-HMG-CoA reductase-associated myopathy: Anti-HMGCR Myopathy Infiltrate. Muscle Nerve. 2015;52(2):189–95.
- Preuße C, Goebel HH, Held J, Wengert O, Scheibe F, Irlbacher K, et al. Immune-Mediated Necrotizing Myopathy Is Characterized by a Specific Th1-M1 Polarized Immune Profile. Am J Pathol. 2012 Dec 1;181(6):2161–71.
- Rojana-udomsart A, Mitrpant C, Bundell C, Price L, Luo Y-B, Fabian V, et al. Complement-mediated muscle cell lysis: A possible mechanism of myonecrosis in anti-SRP associated necrotizing myopathy (ASANM). J Neuroimmunol. 2013 Nov 15;264(1):65–70.
- Ashton C, Junckerstorff R, Bundell C, Hollingsworth P, Needham M. Treatment and outcomes in necrotising autoimmune myopathy: an australian perspective. Neuromuscul Disord [Internet]. 2016 Sep 2 [cited 2016 Sep 16];0(0). Available from: /article/S0960-8966(16)30286-3/
- Allenbach Y, Drouot L, Rigolet A, Charuel JL, Jouen F, Romero NB, et al. Anti-HMGCR Autoantibodies in European Patients With Autoimmune Necrotizing Myopathies: Inconstant Exposure to Statin. 2014;93(3)150-7.

