Abstract
The diagnosis of Susac’s syndrome has been facilitated greatly by appreciation of distinctive magnetic resonance imaging (MRI) findings. As a result there is now increased recognition of what was once thought to be a very rare disease. The aim of this review is to provide an update on the latest developments in Susac’s syndrome and to highlight the importance of early and aggressive immunotherapy.
Clinical and epidemiologic considerations
Susac’s syndrome is the triad of sensorineural deafness, branch retinal artery occlusions (BRAOs) and encephalopathy. The ‘encephalopathy refers to a range of cerebral manifestations including cognitive impairment, psychiatric disturbance, headache, seizures and focal neurologic deficits. Headache is an important but often under-appreciated symptom that frequently has a migrainous character and may precede other symptoms.1
The onset of Susac’s syndrome is usually between 20 and 40 years but cases as young as 7 and as old as 72 years have been reported.2 Females are affected more commonly than males in a proportion of 3:1. The syndrome is rare but the true prevalence is unknown. Because Susac’s syndrome is commonly misdiagnosed as various conditions including acute disseminated encephalomyelitis, multiple sclerosis (MS), neuro-Behçet’s disease, cerebral vasculitis, temporal arteritis or Cogan’s syndrome3 it may be more prevalent than originally supposed. Moreover, the full triad may take months to evolve or never evolve fully and forme frustes of the disease can frustrate diagnosis.1
In the majority of cases, Susac’s syndrome is a monophasic self-limiting disease that remits after one or two years.1 A polycyclic course has also been described in which patients have exacerbations of symptoms over several years. Rarer still, is the chronic continuous phase characterised by fluctuating symptoms without true periods of remission.
Recently, two clinical subtypes of Susac’s syndrome have been proposed; the so-called ‘encephalopathic form’ with predominant encephalopathy and the ‘recurrent BRAO’ subset with a more prolonged and less severe phenotype.4 The ‘recurrent BRAO’ group have minimal clinical or radiological evidence of cerebral involvement, but as the name suggests, develop recurrent episodes of BRAO which can continue episodically for many years without accrual of neurological deficit.4
Ocular manifestations
A feature of Susac’s syndrome is visual field loss due to BRAOs (Figure 1). Affected patients may be asymptomatic but equally others may be too encephalopathic to notice or report difficulties with vision. If Susac’s syndrome is suspected it is important to perform fluorescein angiography as BRAOs may be diagnostic. Retinal arterial wall atheromatous plaques (Gass plaques) may also be present at mid-arteriolar segments away from retinal artery bifurcations. Arteriolar wall hyperfluorescence indicates areas of active disease and may be considered supporting evidence of retinal vasculopathy.5
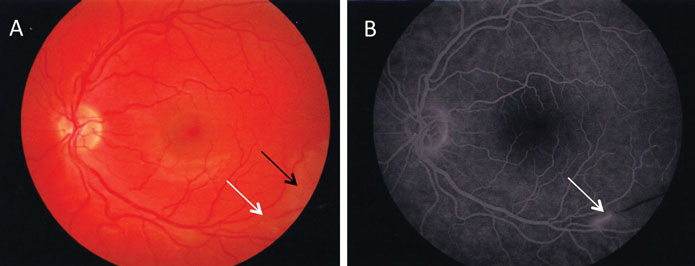
In a recent cross-sectional observational study, nine patients with Susac’s syndrome with retinal involvement were assessed by optical coherence tomography and found to have a significant reduction in retinal nerve fibre layer thickness and macular volume compared to either healthy controls or patients with MS with or without a previous history of optic neuritis.6 The reduction in thickness was present in a sectorial distribution, particularly at the macula, as might be expected with retinal microvascular damage, and different to the generalised thinning seen in MS patients.
Vestibulocochlear involvement
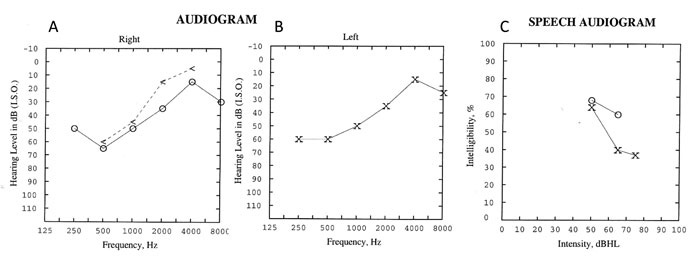
While hearing loss and tinnitus are common in Susac’s syndrome, vestibular symptoms such as vertigo and nausea are also frequent.7 Pure tone audiometry usually reveals bilateral sensorineural hearing loss which is asymmetric and is thought to reflect sequelae of microinfarction in the apical cochlea (Figure 2). Low-to-moderate range frequencies are preferentially affected and poor speech discrimination is common. Vestibular symptoms may be due to peripheral or central vestibular involvement.
Pregnancy
Several authors have noted an association between Susac’s syndrome and pregnancy.8-11 Among eight female patients, most with a new diagnosis of Susac’s syndrome, three developed new BRAOs during pregnancy and three patients experienced a relapse in the post-partum period.8 While this may be due to chance, other autoimmune conditions such as rheumatoid arthritis and thyroid disease may flare in the perinatal period. It is conceivable, therefore, that fluctuations in circulating hormones may be relevant to the underlying mechanism of the disease, perhaps by inducing a hypercoaguable state, and may also be relevant to the overall female preponderance.
Radiological findings
Susac’s syndrome is associated with pathognomonic MRI lesions in the central fibres of the corpus callosum referred to as ‘snowball’ lesions12 (Figure 3). Central callosal ‘icicle’ and ‘spoke’ lesions in contact with the roof of the callosum and a characteristic ‘string of pearls’ appearance of microinfarcts in the internal capsule have also been recognised.4 All of these lesions are best seen on sagittal fluid attenuated inversion recovery (FLAIR) and/or T2 MRI sequences and are often visible on diffusion weighted imaging. Later, as callosal lesions become chronic they assume the appearance of ‘punched out’ holes best seen on T1 sequences (Figure 4). In addition to these findings, punctate subcortical and infratentorial lesions are usually present and these may coalesce (Figure 3). The commonest areas affected are the periventricular regions, centrum semiovale, cerebellum, brainstem and middle cerebellar peduncles. Deep gray matter may be involved. Gray and white matter lesions often enhance (70%) with leptomeningeal enhancement less common4,12.
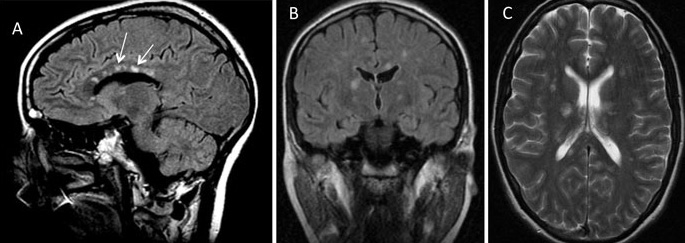
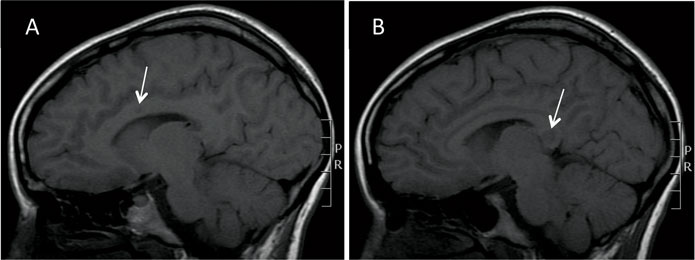
Many patients with Susac’s syndrome are more disabled than their limited lesion load on imaging would indicate. Newer studies using diffusion tensor imaging (DTI) have demonstrated evidence of widespread axonal damage not visible on conventional MRI which may account for this mismatch.13 In particular, microstructural degeneration in the genu of the corpus callosum on DTI appears to be characteristic of Susac’s syndrome. Studies with 7 Tesla MRI have shown that white matter lesions in Susac’s syndrome rarely exhibit a hypointense rim and are less often located in a perivascular location making them morphologically distinct in this regard from the lesions of MS.14
Immunopathogenesis
It is now widely held that Susac’s syndrome occurs as a result of an endotheliopathy of small vessels due to underlying autoimmune dysfunction. Brain biopsy specimens in patients with Susac’s syndrome show areas of microinfarction due to thickening of arteriolar media and loss of capillary networks. While frank vasculitis is not observed, sparse perivascular lymphocytic infiltrates are often described.3
Immunofluorescence studies have identified anti-endothelial cell antibodies (AECAs) in patients with Susac’s syndrome.2,15 Serum from eleven patients with Susac’s syndrome (among whom six of the patients had the full triad) was assessed by indirect immunofluorescence using generic cutaneous microvascular cells. Nine patients were positive for AECAs and eight patients had a characteristic 50-kDa protein detected by western blot that was not present in comparator samples from patients with other autoimmune diseases.
It is not yet clear if AECAs have a pathogenic role in Susac’s syndrome or are merely an epiphenomenon. AECAs are not specific to Susac’s syndrome but have been identified in other autoimmune diseases including scleroderma, systemic lupus erythematosis and Behcet’s disease.2 In support of an antibody-mediated aetiology for Susac’s syndrome, more than 50% of capillaries in brain biopsy specimens stain strongly for the complement protein C4d2.
Immunotherapy
Susac’s syndrome is sufficiently rare that randomised controlled therapeutic trials have not been possible and treatment is based on the results of physician experience supported by individual reports and case series. There is broad agreement that treatment with high dose corticosteroids should be first line therapy. What is less certain is what other agents should be used in conjunction. Immunomodulatory agents most commonly used include intravenous immunoglobulin (IVIg), plasma exchange, azathioprine, mycophenolate mofetil (MMF), methotrexate, cyclosporin A and cyclophosphamide. Ideally, an agent, or combination of agents, which can improve disability due to acute lesions but is also able to exert a sustained disease modifying effect is desirable.
At present, the choice of a particular drug regimen depends on the clinical picture. There is increasing recognition that early, aggressive treatment of the ‘encephalopathic form’ of Susac’s syndrome is necessary. Initial treatment with intravenous corticosteroids is recommended followed by slowly tapering oral corticosteroids and addition of a steroid sparing agent such as MMF. Both IVIg and cyclophosphamide are also suggested on a monthly basis for at least six months.4,5 Maintenance immunomodulatory treatment may be necessary for at least two years from remission11.
For patients in the ‘recurrent BRAO’ group, less aggressive therapy may be warranted as the clinical sequelae are usually less significant.4 In this group, treatment is the same as in the ‘encephalopathic form’ except that oral corticosteroid taper is more rapid and that cyclophosphamide can be withheld in favour of monthly IVIg alone for six months. Patients with a first ever episode of BRAO should probably be treated aggressively from the outset as their disease subtype remains undeclared and the potential neurological sequelae of a relapse may be devastating meaning that the potential benefits arguably outweigh the risks of immunomodulatory therapy.
For patients that deteriorate, continue to relapse, or are intolerant of this regimen then treatment with monoclonal antibodies has been attempted with some success. It may be that these agents will become the preferred therapeutic option as an adjunct to corticosteroids for early aggressive treatment of Susac’s syndrome as further data emerge. Specifically, there is growing experience with the anti CD20 monoclonal antibody, Rituximab in Susac’s syndrome.4,5,10 The tumour necrosis factor (TNF) inhibitor, Infliximab may also be beneficial.9 Both of these agents have efficacy in juvenile dermatomyositis, a condition believed to share a similar immunopathogenesis to Susac’s syndrome.5,15
Treatment of hearing loss and tinnitus
Intratympanic injection of dexamethasone in the acute phase of hearing loss and tinnitus may provide transient benefit and may help justify more aggressive immunotherapy on the grounds of potential for reversibility. In those patients with profound sensorineural hearing loss consideration of cochlear implants is warranted7.
Treatment of ocular manifestations
The aim of treatment of visual deficits is prevention of further damage with immunotherapy as, once established, retinal infarcts are permanent. Hyperbaric oxygen may improve acute onset visual symptoms in Susac’s syndrome16. Neovascularisation resulting from retinal ischaemia has been treated with laser photocoagulation with some success8.
Prognosis
Prognosis is to some extent determined by whether patients have an ‘encephalopathic form’ of Susac’s syndrome or a clinical picture more in keeping with a ‘recurrent BRAO’ subtype (see Clinical and Epidemiologic Considerations and Immunotherapy). Prior to the recognition of the need for aggressive treatment of the encephalopathic form of Susac’s syndrome, approximately 50% of patients suffered ongoing cognitive impairment.1 However, if treated early, many patients with Susac’s syndrome are now able to make an excellent recovery despite significant encephalopathy at presentation.
Conclusion
Susac’s syndrome is a rare but likely underdiagnosed condition. Recent research developments have led to improved diagnosis and growing insights into its underlying immunopathogenesis. Treatment with early aggressive immunotherapy is warranted in many cases and a role for monoclonal antibody therapy is emerging as an adjunct to more traditional immunosuppression.
References
- Susac J. Susac’s syndrome: The triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology. 1994;44:591-593.
- Magro CM, Poe JC, Lubow M, Susac JO. Susac syndrome: an organ-specific autoimmune endotheliopathy syndrome associated with anti-endothelial cell antibodies. Am J Clin Pathol. 2011 Dec;136(6):903-12.
- Dörr J, Radbruch H, Bock M, Wuerfel J, Brüggemann A, Wandinger KP, Zeise D, Pfueller CF, Zipp F, Paul F. Encephalopathy, visual disturbance and hearing loss-recognizing the symptoms of Susac syndrome. Nat Rev Neurol. 2009 Dec;5(12):683-8.
- Rennebohm R, Susac JO, Egan RA, Daroff RB. Susac’s Syndrome–update. J Neurol Sci. 2010 Dec 15;299(1-2):86-91.
- Rennebohm R, Egan R, Susac J. Treatment of Susac’s syndrome. Curr Treat Options Neurol. 2008;10:67-74.
- Brandt AU, Zimmermann H, Kaufhold F, Promesberger J, Schippling S, Finis D, Aktas O, Geis C, Ringelstein M, Ringelstein EB, Hartung HP, Paul F, Kleffner I, Dörr J. Patterns of retinal damage facilitate differential diagnosis between Susac syndrome and MS. PLoS One. 2012;7(6):e38741.
- Roeser MM, Driscoll CL, Shallop JK, Gifford RH, Kasperbauer JL, Gluth MB. Susac syndrome–a report of cochlear implantation and review of otologic manifestations in twenty-three patients. Otol Neurotol. 2009 Jan;30(1):34-40.
- Aubart-Cohen F, Klein I, Alexandra J, Bodaghi B, Doan S, Fardeau C, Lavallée P, Piette J, Hoang P, Papo T. Long-term outcome in Susac syndrome. Medicine. 2007;86:93-102.
- Hardy TA, Garsia RJ, Halmagyi GM, Lewis SJ, Harrisberg B, Fulham MJ, Barnett MH. Tumour necrosis factor (TNF) inhibitor therapy in Susac’s syndrome. J Neurol Sci. 2011 Mar 15;302(1-2):126-8.
- Mateen FJ, Zubkov AY, Muralidharan R, Fugate JE, Rodriguez FJ, Winters JL, Petty GW. Susac syndrome: clinical characteristics and treatment in 29 new cases. Eur J Neurol. 2012 Jun;19(6):800-11.
- Deane KD, Tyler KN, Johnson DW, Tanabe JL, Oskarrson BE, Nitka EE, Brass E, Davies JK, Striebich CC. Susac syndrome and pregnancy: disease management. J Clin Rheumatol. 2011 Mar;17(2):83-8.
- Susac J, Murtagh F, Egan R, Berger J, Bakshi R, Lincoff N, Gean A, Galetta S, Fox R, Costello F, Lee A, Clark J, Layzer R, Daroff R. MRI findings in Susac’s syndrome. Neurology. 2003;61:1783-1787.
- Kleffner I, Deppe M, Mohammadi S, Schwindt W, Sommer J, Young P, Ringelstein EB. Neuroimaging in Susac’s syndrome: focus on DTI. J Neurol Sci. 2010 Dec 15;299(1-2):92-6.
- Wuerfel J, Sinnecker T, Ringelstein EB, Jarius S, Schwindt W, Niendorf T, Paul F, Kleffner I, Dörr J. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012 Nov;18(11):1592-9.
- Jarius S, Neumayer B, Wandinger K, Hartmann M, Wildemann B. Anti-endothelial serum antibodies in a patient with Susac’s syndrome. J Neurol Sci. 2009;285:259-261.
- Li HK, Dejean BJ, Tang RA. Reversal of visual loss with hyperbaric oxygen treatment in a patient with Susac syndrome. Ophthalmology. 1996 Dec;103(12):2091-8.
