Introduction

Stuart Viegas is Editor of ACNR’s myasthenia gravis (MG) series. He is a Consultant Neurologist who leads the Imperial MG and muscle service, UK. He qualified from UCL medical school before training in Wessex and London. He did his research in MG in Oxford under the supervision of Professor Angela Vincent. He is involved in clinical research projects and continues to support MG patients through the Myaware charity.
Conflict of Interest Statement: Stuart Viegas is married to Ann Donnelly, the Co-Editor of ACNR. This article has been subject to our normal peer review process, being peer reviewed by two expert, external reviewers prior to acceptance by Todd Hardy, Co-Editor of ACNR.
I was delighted to be asked by ACNR’s editorial team to help bring a series of articles on myasthenia gravis (MG) to the wider neuroscience community. I hope that the experienced authors in the field will help cover a range of topics that its readers will find stimulating and educational, but most importantly clinically relevant.
Compared with many neurological conditions, MG is a relatively rare but important antibody mediated disease. It is one that all neurologists need to be familiar with. Prompt recognition of the cardinal symptoms – the characteristic pattern of fluctuation and variability should trigger urgent assessment and investigation in all suspected cases. Successful treatment with pyridostigmine, often with good symptomatic improvement, is something most patients will remember for many years.
Early identification of patients who will have a challenging or refractory disease course and the need for further treatment remains a challenge. MG is heterogenous – with pure ocular and generalised cases; the latter ranging from minimal manifestations to life-threating complications. Myasthenic crises are a neurological emergency requiring multidisciplinary input and early involvement of our intensive care colleagues. Timely recognition, good supportive care and effective treatment with plasma exchange and/or intravenous immunoglobulin has led to a significant reduction in mortality across all age groups. Seeing patients responding to such treatment is incredibly rewarding for the treating physician and helps consolidate the doctor-patient relationship.
Our increased understanding of the physiological disturbances and immune mediated mechanisms underlying MG has led to the developments of improved antibody assays, neurophysiology techniques and chest imaging that is critical for ensuring accurate diagnosis. Treatment regimes based around pyridostigmine, corticosteroids, steroid sparing drugs, thymectomy and the emergency treatments outlined above have been used for many years.
The ABN guidelines published in 2015 aimed to disseminate the knowledge and experience of specialists in the field to the wider neurology community and were well received. Reflecting the clinical heterogeneity, the response to standard treatment is also highly variable. The antibody subtypes may respond to different therapies and this information, together with age and comorbidities, influences treatment selection.
In contrast with other neuroimmunological disorders such as multiple sclerosis, there has been a paucity of new treatments until the last few years. Novel biological therapies targeting B cells, T cells and complement pathways have since been developed, trialled and now introduced into clinical practice which makes it an exciting time to be working in the field.
Given the changing landscape with respect to MG treatments, we will start the series with Dr Jennifer Spillane and her overview of the new drugs that have arrived, those on the horizon and those still in the research arena. MG, like many autoimmune disorders, often affects young women and its management in pregnancy warrants special attention. In the second article Dr Georgina Burke will discuss pre-conception planning, antenatal and postnatal care which requires close multidisciplinary liaison with our obstetric medicine and anaesthetic colleagues.
Whilst the incidence of MG in young women has remained static for many years, there is an increasing number of older onset cases, often with a male predominance. Older onset patients with their co-morbidities and polypharmacy require a different management approach. Furthermore, the younger onset patients get older and may also have been exposed to long term immunosuppression and corticosteroid use. Associate Professor Isabel Leite will outline the management of this important patient cohort further in the third article.
Finally, all neurologists looking after MG patients should be aware of some important cardiothoracic and oncological issues relating to the thymus and thymic malignancy which will be described further by Mr Dio Stavroulias and Dr Joanne Evans in the concluding article.
Myasthenia Gravis – a new era of treatment options
Abstract
Myasthenia Gravis (MG) is an autoimmune disease of the neuromuscular junction that causes fluctuating fatigable neuromuscular weakness. The spectrum of severity ranges from mild intermittent ptosis to respiratory failure requiring ventilatory support.
There are various treatment options. Pyridostigmine is the first line treatment but provides symptomatic relief only. The mainstay of treatment rests on immunosuppression using corticosteroids and non-specific immunosuppressant agents such as azathioprine, mycophenolate mofetil and methotrexate. Intravenous immunoglobulin and plasma exchange are generally reserved for acute severe exacerbations. Thymectomy is also an option for some patients.
Although a large proportion of patients with MG achieve disease control with these treatments, others have refractory disease with ongoing symptoms, frequent exacerbations and dependence on rescue therapies. Other patients are exposed to long term high dose steroids.
Increased understanding of the pathogenesis of MG has led to the development of newer agents with a more specific mechanism of action and a rapid onset of effect. These novel targets include B cells, the complement cascade and the neonatal Fc receptor. Other potential targets include cell surface receptors, chemokines and cytokines.
In this paper we review the evidence for these newer therapies and discuss where they may fit into the treatment paradigm for patients with MG.
Introduction
Myasthenia Gravis (MG) is the archetypical neurological autoimmune disease, characterised by fluctuating fatigable weakness. Although 80% of patients present with ocular symptoms, most will go on to develop generalised disease with a 10-15% lifetime risk of Myasthenic Crisis. The epidemiology of MG is interesting with a bimodal peak- one peak in early adulthood and a second peak in older age.
80% of patients with MG have antibodies to the acetylcholine receptor (AChR). These antibodies are divalent and are of the IgG1 and IgG3 subtypes. They exert their effect by three different mechanisms: cross-linking and internalisation of AChRs, activation of complement leading to formation of the membrane attack complex and subsequent damage to the neuromuscular junction, and functional block of AChRs.
Whilst 15-20% of patients with AChR MG have a thymoma, the thymus in younger patients with AChR antibody positive MG tends to be hyperplastic and infiltrated with inflammatory cells.
Approximately 5-10% of patients with MG have antibodies to Muscle Specific Tyrosine Kinase (MuSK), an important post-synaptic clustering protein. In contrast to AChR antibody positive MG, MuSK antibodies are of the IgG4 subtype, are not divalent and do not activate complement. Antibodies against LRP-4, another post-synaptic protein, are detected in a small number of patients with MG.
The remaining 10% of patients do not have antibodies detectable by conventional assays and are termed seronegative. However about 30% of this cohort will have antibodies to AChRs detected using a more specific cell-based assay [1]. If an antibody is present, it’s important to detect it – not only to confirm the diagnosis but also to guide treatment as we enter an era of more targeted specific management.
Current Treatment
The treatment of MG can be thought of as symptomatic, immunosuppressant or immunomodulatory.
Pyridostigmine, an acetylcholinesterase inhibitor is still the first line treatment for most patients with MG. It provides symptomatic relief in a proportion of patients but has no disease modifying effect.
Thymectomy has been used for the management of MG since the 1930s. It is almost always indicated for patients with thymoma, and a randomised controlled trial showed that patients with non-thymomatous MG under the age of 65 had better MG control with lower steroid dose following thymectomy [2]. It is not thought to benefit patients with MuSK antibody-positive MG.
IVIg and plasma exchange are generally reserved for patients with acute deteriorations though a minority of patients remain dependent on these treatments in the longer term.
The management of MG however largely rests on non-specific broad-spectrum immunosuppression using steroids and a range of non-steroidal immunosuppressant agents such as azathioprine, mycophenolate and methotrexate with others, such as ciclosporin and tacrolimus used less frequently. Non-steroidal therapies often have a slow onset of action, and patients can therefore be exposed to high dose steroids for many months before symptoms come under control.
Although symptoms in most MG patients are eventually adequately controlled on the current available therapies, over 10% of patients are refractory [3] and real world studies show that over 40% of patients have unacceptable disease control as measured by a Myasthenia Gravis Activities of Daily Living (MG ADL) score greater than three [4].
There has therefore been a longstanding need for specific, targeted treatments that are more efficacious, with a faster onset of action. Advances in the understanding of the pathogenesis of MG have unveiled a number of new treatment targets which have the potential to herald a new era for the management of patients with MG.
Alternative Approaches
MG is a T cell dependent, B cell mediated auto-immune disease and there are various targets that could be explored to develop treatments for MG.
For the purposes of this review we will focus on the treatments that are either in regular clinical use or are close to clinical use – namely B cell depleting therapy, complement inhibition, and inhibition of IgG recycling by targeting the neonatal Fc receptor.
Anti B cell therapy
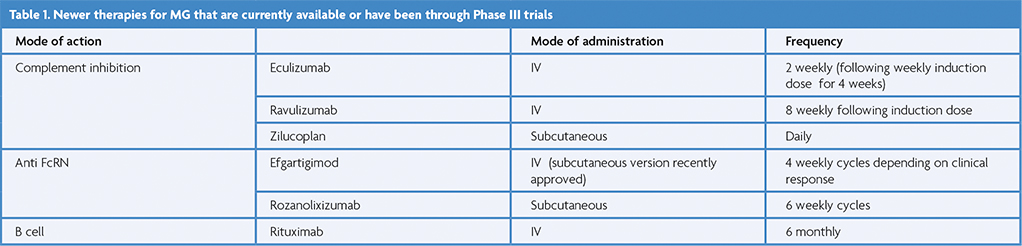
Inhibiting the antibody producing B cells is a rational approach to treating antibody mediated autoimmune disease. Rituximab is humanised anti-CD20 monoclonal antibody that depletes memory B cells but not long-lived plasma cells. There have been retrospective observational and single armed reports regarding the use of Rituximab in MG for many years, as well as systematic reviews and meta-analyses that have suggested a positive effect in MG [5]. It was therefore disappointing that a randomised placebo-controlled Phase II trial investigating the steroid-sparing effect of Rituximab was negative in 2021 [6]. Analysing this trial, a number of features stand out: there was a high placebo effect, the steroid dose was low at 20mg, meaning that a change could not be easily detected and a significant proportion of the patients had relatively mild disease. The efficacy of Rituximab in generalised AChR MG therefore remains uncertain. One possibility is that Rituximab is more effective in recent onset MG as was suggested in the recent RINOMAX trial. This blinded placebo-controlled study examined the use of low dose Rituximab in a cohort of patients with newly diagnosed MG and found that Rituximab treatment resulted in a higher proportion reaching minimal manifestations at 16 weeks with less requirement for rescue treatment [7]. Further follow up is required to see the longer-term outcome in this cohort.
Given the disappointing results of Rituximab particularly in refractory AChR MG, there has been a search for other B Cell depleting agents that may act against long lived plasma cells. A Phase 3 trial is currently underway into Inebilizumab, a humanised monoclonal antibody against CD19 which in contrast to Rituximab depletes a wide range of B cells including plasma cells and plasmablasts (NCT04524273).
In contrast to AChR MG, Rituximab has been shown to be very effective in MuSK MG with a blinded multi centre prospective review showing a statistically significant impact on steroid usage and MG symptoms [8].
Complement inhibition
The classical complement pathway is implicated in the pathogenesis of AChR MG: formation of the membrane attack complex leads to destruction of the neuromuscular end-plate. Targeting complement is therefore a potential treatment to mitigate the pathogenic effects of AChR antibodies in MG.
Eculizumab, a humanised anti-C5 monoclonal antibody was the first complement inhibitor trialled in MG and examined its efficacy in patients with refractory generalised MG – the primary endpoint of improvement in ADL compared to placebo was not met but secondary endpoints of significant improvements in ADL and QMG were and open label extension data were encouraging [9].
Having shown that complement inhibition might be effective in MG – investigators subsequently sought to develop more convenient routes of administration as the requirement for fortnightly IV infusions of Eculizumb have limited its more widespread use.
Ravulizumab is a C5-inhibitor that has been engineered to have an extended half-life and is given as an IV infusion every 8 weeks. The CHAMPION trial met its primary endpoint; Ravulizumab treated patients had improvement in their MG ADL score at 26 weeks compared to standard care [10].
A further complement inhibitor is Zilucoplan, a 15 amino acid cyclic peptide that inhibits the complement cascade by binding to C5a and C5B with high affinity – it is given as a daily subcutaneous injection and its efficacy was established in the RAISE trial which showed a significant improvement MG ADL score at 12 weeks [11].
Both Eculizumab and Ravulizumab are approved by the FDA and the EMA. Eculizumab is approved but not reimbursed in the UK.
Other complement inhibitors are under investigation in Phase 2 or 3 trials including Pozelimab (usually given in combination with Cemdisiran which acts through RNA interference), Gefurlimab and Vemircopan (NCT05070858, NCT05556096, NCT05218096).
The onset of action in all complement inhibitor trials studied so far seems to be rapid. Overall, complement inhibition seems to be well tolerated though the route of administration – either IV or subcutaneous injection – may be a limiting factor for some patients. Complement inhibition is associated with a risk of Neisseria meningitides septicaemia, so all patients must be vaccinated accordingly.
Complement inhibition will be reserved for patients with AChR antibody positive disease – as previously discussed, the complement cascade is not implicated in the pathogenesis of MuSK MG.
Anti FcRN therapies
The neonatal FC receptor (FcRN) is widely distributed in many cells and has an important role in the recycling of IgG, thus prolonging its half-life.
Blocking of the FC receptor interferes with this recycling and reduces the plasma concentration of IgG. By reducing the level of IgG, the effect of the pathogenic AChR antibodies in MG can be reduced, and thus FcRN blocking can be thought of as similar to ‘medical plasma exchange’. The serum levels of other immunoglobulins do not seem to be affected by FcRN inhibition,
Various FcRN blocking agents have been investigated or are currently undergoing Phase 3 trials for the treatment of generalised MG.
Efgartigimod is an engineered Fc fragment of human IgG1 that binds to FC receptors with greater affinity than human IgG, thus inhibiting its recycling.
The Phase 3 ADAPT study showed that Efgartigimod was effective in improving MG ADL scores compared to placebo [12]. It was given as a cyclical treatment – a weekly IV infusion for 4 weeks followed by a variable treatment schedule depending on symptom re-emergence.
Efgartigimod is licensed by the MHRA, EMA and FDA and has been available in the UK under an Early Access to Medicine Scheme since May 2022.
A subcutaneous version has just been licensed by the US and different dosing schedules are currently being investigated.
Rozanolixizumab is a high affinity humanised IgG4 monoclonal antibody against FcRn and is administered by weekly subcutaneous injection. The Phase 3 MyCarin study showed a significant improvement in Mg ADL scores, and in contrast to other FcRN treatments is currently approved in the UK and Europe for the treatment of both AChR and MuSK MG [13].
Other FcRN therapies – Batoclimab and Nicolimab are currently undergoing Phase III trials (NCT05403541, NCT04951622).
Anti-FcRN treatments tend to be well tolerated with minor infections and headache being the most reported side effects. In contrast to complement treatment, there is scientific rationale for FcRN use in MuSK positive MG. The onset of action of FcRN treatment is fast. Repeated dosing is necessary with an average inter-treatment interval of 7 weeks though some patients require more frequent treatment.
Other therapeutic targets
Cell-surface receptors, Cytokines and chemokines
The signalling molecules that help in coordination of the immune response and upregulation of B and T cells are potential targets in MG.
CD40 is a molecule expressed on B cells and is important for differentiation and activation of memory B cells and through its interaction with CD154, is essential for T cell dependent antibody responses. Isacalimab is a monoclonal antibody that targets CD40 and is undergoing a phase II trial in MG currently (NCT0256576).
IL6 has a role in T and B cell signalling pathways and in the differentiation of B cells. Satralizumab has been successful in phase 3 trials for treatment of Neuromyelitis Optica Spectrum Disorder and currently is undergoing investigation for the treatment of MG (NCT04963270). Tocilizumab, which also inhibits IL6 signalling has been found to be beneficial in MG in case reports and a single arm open label study of six patients [14,15].
Bruton’s Tyrosine Kinase (BTK) has a role in B cell proliferation and differentiation and is a potential target in MG. Tolebrutinib is an oral BTK inhibitor and has been studied in MS. However, a phase III study was suspended earlier this year because of hepatotoxicity.
B cell activating factor (BAFF) is upregulated in MG – Belimumab, an anti BAFF agent has been studied in Systemic Lupus Erythematosus but a phase 2 trial did not show any clear benefit for its use in MG [16].
Other potential targets include plasma cells with anti CD38 agents such as Daratumumab which is used in Myeloma, and cytokines that target T cell activation such as IL17, IL12 and IL23 but these have not been studied in MG in detail to date.
Alternative strategies
CAR-T cell therapy
Chimeric antigen receptor (CAR) T cell therapy has shown remarkable efficacy in haematological malignancy in recent years.
CAR-T cell therapy could in principle be adapted for autoimmune diseases by using an engineered T cell which could bind to a pathogenic B cell, thus specifically targeting pathogenic cells – so called chimeric autoantibody receptor T Cell therapy.
Haematopoietic Stem cell transplant
‘Resetting’ of the immune system with haematopoietic stem transplantation has been investigated in several refractory autoimmune diseases. The process involves stimulation of haematopoietic stem cell production, cell harvesting followed by bone marrow ablation and reinfusion of the harvested cells. A retrospective case series reported remarkable benefit with patients having remission of their MG [17]. However, the potential for adverse events means that few patients have undergone this treatment.
Questions and future directions
The landscape of MG treatment is clearly changing. Rather than slow acting non-specific treatments we now have more defined targets for treatments with a faster onset of action. The agents described were mostly not trialled on selected refractory patients, so where these newer therapies should fit into the treatment paradigm amongst the familiar agents remains uncertain. Thus far they have been generally licensed as add on treatments, but how early they should be used and in what severity of MG is unknown. The considerable benefits in terms of efficacy, speed of onset, and reduction in steroid use must be balanced against their cost and the lack of long-term safety data.
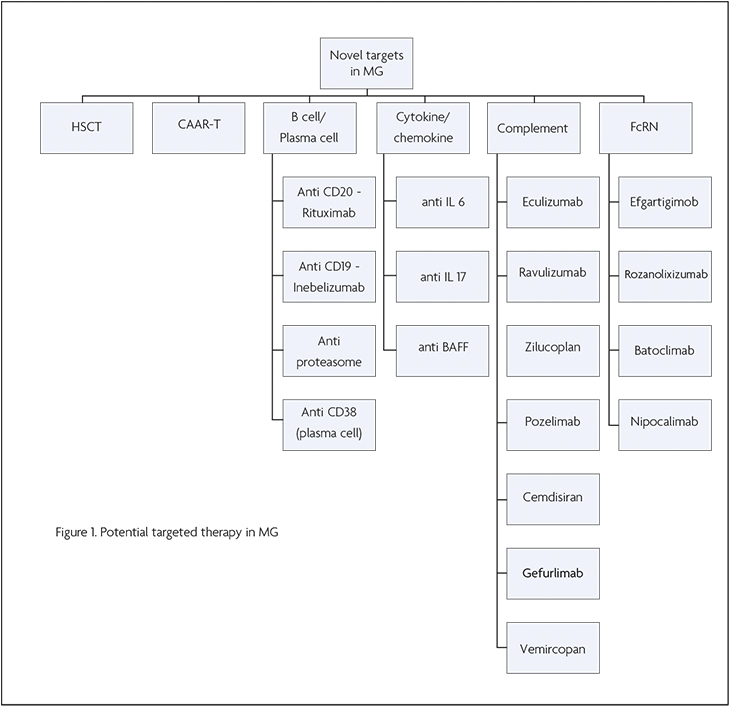
Figure 1 Potential targeted therapy in MG
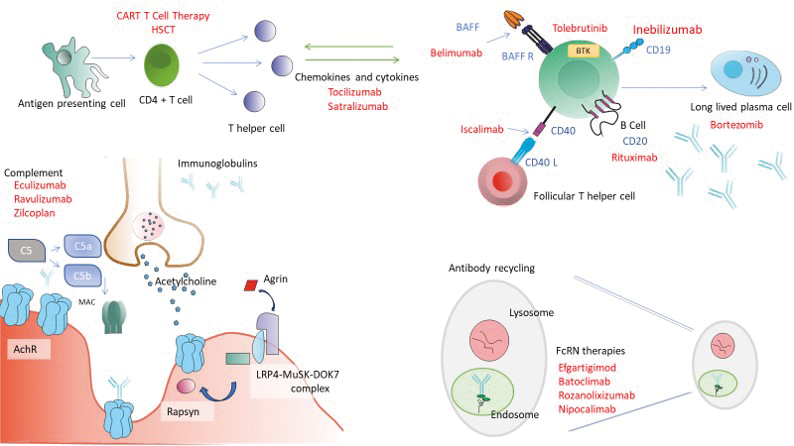
Figure 2. Schematic diagram depicting the site of action of novel Myasthenia Gravis treatments.
Top left – Antigen presenting cells interact with CD4 positive T cells which, under the influence of certain cytokines and other activating factors leads to B cell proliferation. B cells differentiate into antibody producing memory B cells and long lived plasma cells. This pathway can be targeted at different levels. Rituximab (anti CD20) and Inebilizumab (anti CD19) target B cells directly, Isacalimab blocks the CD40 signalling pathway which is important for differentiation and activation of memory B cells. Cytokines such as IL6 are important for B cell differentiation and T cell regulation. Tocilizumab and Satralizumab are anti IL6 monoclonal antibodies. Other targets such as B Cell activating factor (BAFF) or Bruton’s Tyrosine Kinase (BK) with agents such as Belimumab and Tolebrutinib affect the B cell pathway indirectly. Bortezomib inhibits long lived plasma cells.
Bottom left – the neuromuscular junction (NMJ) and the clustering pathway of Acetylcholine receptors (AchRs). Agrin is secreted at the nerve terminal into the synaptic cleft. This interacts with the Muscle Specific Kinase (MuSK) – low density lipoprotein receptor-related protein 4 (LRP4) complex which is located on the post synaptic membrane which in turn activated Rapsyn.
Complement activation is an important pathogenic mechanism for IgG1 and IgG3 AChR antibodies. The conversion of C5 to C5a and C5b results in the formation of the membrane attack complex. Complement inhibitors such as Eculizumab, Ravulizumab and Zilcoplan interrupt this pathway.
Bottom left – IgG is recycled in the endosomal-lysosomal system. Binding of IgG to the FcrN protects it from lysosomal degradation. Anti FcRN therapies such as Efgartigimod, Rozanolixizumab, Nipocalimab and Batoclimab bind competitively to the FcRN thus preventing IgG recycling.
Read the next article in this series: Myasthenia Gravis and pregnancy | ACNR
References
- Damato V, Spagni G, Monte G, Woodhall M, Jacobson L, Falso S, Smith T, Iorio R, Waters P, Irani SR, Vincent A, Evoli A. Clinical value of cell-based assays in the characterisation of seronegative myasthenia gravis. J Neurol Neurosurg Psychiatry. 2022 Sep;93(9):995-1000. Epub 2022 Jul 14. PMID: 35835469. https://doi.org/10.1136/jnnp-2022-329284
- Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, Ströbel P, Mazia C, Oger J, Cea JG, Heckmann JM, Evoli A, Nix W, Ciafaloni E, Antonini G, Witoonpanich R, King JO, Beydoun SR, Chalk CH, Barboi AC, Amato AA, Shaibani AI, Katirji B, Lecky BR, Buckley C, Vincent A, Dias-Tosta E, Yoshikawa H, Waddington-Cruz M, Pulley MT, Rivner MH, Kostera-Pruszczyk A, Pascuzzi RM, Jackson CE, Garcia Ramos GS, Verschuuren JJ, Massey JM, Kissel JT, Werneck LC, Benatar M, Barohn RJ, Tandan R, Mozaffar T, Conwit R, Odenkirchen J, Sonett JR, Jaretzki A 3rd, Newsom-Davis J, Cutter GR; MGTX Study Group. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med. 2016 Aug 11;375(6):511-22. Erratum in: N Engl J Med. 2017 May 25;376(21):2097]. PMID: 27509100; PMCID: PMC5189669. https://doi.org/10.1056/NEJMoa1602489
- Rath J, Brunner I, Tomschik M, Zulehner G, Hilger E, Krenn M, Paul A, Cetin H, Zimprich F. Frequency and clinical features of treatment-refractory myasthenia gravis. J Neurol. 2020 Apr;267(4):1004-1011. Epub 2019 Dec 11. PMID: 31828474; PMCID: PMC7109164. https://doi.org/10.1007/s00415-019-09667-5
- Petersson M, Feresiadou A, Jons D, Ilinca A, Lundin F, Johansson R, Budzianowska A, Roos AK, Kågström V, Gunnarsson M, Sundström P, Piehl F, Brauner S. Patient-Reported Symptom Severity in a Nationwide Myasthenia Gravis Cohort: Cross-sectional Analysis of the Swedish GEMG Study. Neurology. 2021 Aug 10;97(14):e1382-91. Epub ahead of print. Erratum in: Neurology. 2021 Dec 14;97(24):1141. PMID: 34376512; PMCID: PMC8520390. https://doi.org/10.1212/WNL.0000000000012604
- Li T, Zhang GQ, Li Y, Dong SA, Wang N, Yi M, Qi Y, Zhai H, Yang L, Shi FD, Yang CS. Efficacy and safety of different dosages of rituximab for refractory generalized AChR myasthenia gravis: A meta-analysis. J Clin Neurosci. 2021 Mar;85:6-12. Epub 2021 Jan 2. PMID: 33581791. https://doi.org/10.1016/j.jocn.2020.11.043
- Nowak RJ, Coffey CS, Goldstein JM, Dimachkie MM, Benatar M, Kissel JT, Wolfe GI, Burns TM, Freimer ML, Nations S, Granit V, Smith AG, Richman DP, Ciafaloni E, Al-Lozi MT, Sams LA, Quan D, Ubogu E, Pearson B, Sharma A, Yankey JW, Uribe L, Shy M, Amato AA, Conwit R, O’Connor KC, Hafler DA, Cudkowicz ME, Barohn RJ; NeuroNEXT NN103 BeatMG Study Team. Phase 2 Trial of Rituximab in Acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis: The BeatMG Study. Neurology. 2021 Dec 2;98(4):e376-89. Epub ahead of print. PMID: 34857535; PMCID: PMC8793103. https://doi.org/10.1212/WNL.0000000000013121
- Piehl F, Eriksson-Dufva A, Budzianowska A, Feresiadou A, Hansson W, Hietala MA, Håkansson I, Johansson R, Jons D, Kmezic I, Lindberg C, Lindh J, Lundin F, Nygren I, Punga AR, Press R, Samuelsson K, Sundström P, Wickberg O, Brauner S, Frisell T. Efficacy and Safety of Rituximab for New-Onset Generalized Myasthenia Gravis: The RINOMAX Randomized Clinical Trial. JAMA Neurol. 2022 Nov 1;79(11):1105-1112. PMID: 36121672; PMCID: PMC9486640 https://doi.org/10.1001/jamaneurol.2022.2887
- Hehir MK, Hobson-Webb LD, Benatar M, Barnett C, Silvestri NJ, Howard JF Jr, Howard D, Visser A, Crum BA, Nowak R, Beekman R, Kumar A, Ruzhansky K, Chen IA, Pulley MT, LaBoy SM, Fellman MA, Greene SM, Pasnoor M, Burns TM. Rituximab as treatment for anti-MuSK myasthenia gravis: Multicenter blinded prospective review. Neurology. 2017 Sep 5;89(10):1069-1077. Epub 2017 Aug 11. PMID: 28801338. https://doi.org/10.1212/WNL.0000000000004341
- Howard JF Jr, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, Jacob S, Vissing J, Burns TM, Kissel JT, Muppidi S, Nowak RJ, O’Brien F, Wang JJ, Mantegazza R; REGAIN Study Group. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017 Dec;16(12):976-986. https://doi.org.10.1016/S1474-4422(17)30369-1. Epub 2017 Oct 20. Erratum in: Lancet Neurol. 2017 Dec;16(12):954. PMID: 29066163.
- Vu T, Meisel A, Mantegazza R, Annane D, Katsuno M, Aguzzi R, Enayetallah A, Beasley KN, Rampal N, Howard JF Jr, for the CHAMPION MG Study Group. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. NEJM Evid. 2022.https://doi.org/10.1056/EVIDoa2100066
- Howard JF Jr, Bresch S, Genge A, Hewamadduma C, Hinton J, Hussain Y, Juntas-Morales R, Kaminski HJ, Maniaol A, Mantegazza R, Masuda M, Sivakumar K, Śmiłowski M, Utsugisawa K, Vu T, Weiss MD, Zajda M, Boroojerdi B, Brock M, de la Borderie G, Duda PW, Lowcock R, Vanderkelen M, Leite MI; RAISE Study Team. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. 2023 May;22(5):395-406. PMID: 37059508. https://doi.org/10.1016/S1474-4422(23)00080-7
- Howard JF Jr, Bril V, Vu T, Karam C, Peric S, Margania T, Murai H, Bilinska M, Shakarishvili R, Smilowski M, Guglietta A, Ulrichts P, Vangeneugden T, Utsugisawa K, Verschuuren J, Mantegazza R; ADAPT Investigator Study Group. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021 Jul;20(7):526-536. https://doi.org/10.1016/S1474-4422(21)00159-9. Erratum in: Lancet Neurol. 2021 Aug;20(8):e5. PMID: 34146511.
- Bril V, Drużdż A, Grosskreutz J, Habib AA, Mantegazza R, Sacconi S, Utsugisawa K, Vissing J, Vu T, Boehnlein M, Bozorg A, Gayfieva M, Greve B, Woltering F, Kaminski HJ; MG0003 study team. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023 May;22(5):383-394. PMID: 37059507. https://doi.org/10.1016/S1474-4422(23)00077-7
- Jonsson DI, Pirskanen R, Piehl F. Beneficial effect of tocilizumab in myasthenia gravis refractory to rituximab. Neuromuscul Disord. 2017 Jun;27(6):565-568. Epub 2017 Mar 16. PMID: 28433474. https://doi.org/10.1016/j.nmd.2017.03.007
- Jia D, Zhang F, Li H, Shen Y, Jin Z, Shi FD, Zhang C. Responsiveness to Tocilizumab in Anti-Acetylcholine Receptor-Positive Generalized Myasthenia Gravis. Aging Dis. 2023 Jul 6. Epub ahead of print. PMID: 37450932. https://doi.org/10.14336/AD.2023.0528
- Hewett K, Sanders DB, Grove RA, Broderick CL, Rudo TJ, Bassiri A, Zvartau-Hind M, Bril V; BEL115123 Study Group. Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis. Neurology. 2018 Apr 17;90(16):e1425-e1434. Epub 2018 Mar 21. PMID: 29661905; PMCID: PMC5902787. https://doi.org/10.1212/WNL.0000000000005323
- Bryant A, Atkins H, Pringle CE, Allan D, Anstee G, Bence-Bruckler I, Hamelin L, Hodgins M, Hopkins H, Huebsch L, McDiarmid S, Sabloff M, Sheppard D, Tay J, Bredeson C. Myasthenia Gravis Treated With Autologous Hematopoietic Stem Cell Transplantation. JAMA Neurol. 2016 Jun 1;73(6):652-8. PMID: 27043206. https://doi.org/10.1001/jamaneurol.2016.0113


