Di-methyl fumarate (Tecfidera™)
Introduction
In the first and second part of this three-part series of articles, we looked at the sphingosine-1-phosphate receptor modulator fingolimod and the pyrimidine synthesis inhibitor teriflunomide respectively. Dimethyl fumarate (DMF, Tecfidera®), the most recent oral therapy to be approved, will be the subject of this final article.
In January 2014 the European Medicine Agency (EMA) licenced DMF for the treatment of adult patients with relapsing and remitting multiple sclerosis (RRMS) and in August 2014 NICE recommended DMF as a possible treatment for people with active RRMS that isn’t highly active or rapidly evolving severe RRMS. The starting dose of Tecfidera is 120 mg twice a day, after seven days, the dose is increased to the recommended dose of 240mg twice a day.
Mechanism of Action
The active ingredient of Tecfidera is the oral formulation of dimethyl fumarate C6H8O4 (DMF / BG-12). DMF was developed following the successful use of Fumaderm® (Biogen-Idec, Weston, MA, USA) for psoriasis in Germany.2 Psoriasis like MS is believed to have an autoimmune pathogenesis. The use of fumaric esters in autoimmune conditions is based on the evidence that these compounds activate Nrf2 transcriptional pathway with subsequent upregulation of elements involved in the antioxidant response.3 These effects ultimately result in the inhibition of pro-inflammatory mechanisms and may promote a neuroprotective effect.4
DMF is rapidly cleaved into the active metabolite monomethyl fumarate (MMF) by esterases in the alkaline environment of the intestine. Ingestion with food delays maximal levels from two to five hours, with longer delays seen, the higher the fat content, but the total drug absorbed remains equivalent.5 MMF is metabolised to fumaric acid and citric acid before being broken down to CO2 via the Krebs cycle, as well as glucose, cysteine and acetylcysteine conjugates. Elimination occurs over eight hours with exhalation of CO2 being the primary route accounting for approximately 60% of the Tecfidera dose.6 Renal and faecal elimination are secondary routes of elimination. DMF and MMF seem to have little potential for drug interactions with no significant P450 (CYP450) inhibition or induction and low protein binding.5
Efficacy
DMF has been studied in psoriasis, rheumatoid arthritis, Crohn’s disease, in addition to MS in greater than 18 studies, and for longer than four years in a subset of patients enrolled from the two pivotal RRMS Phase III trials (DEFINE and CONFIRM) into the long-term safety extension study ENDORSE.7-9
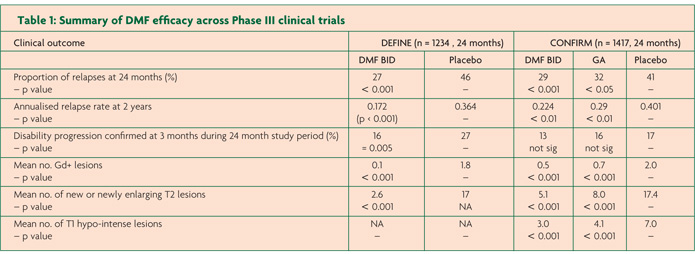
DEFINE and CONFIRM followed the Phase II study which revealed significant anti-inflammatory activity in RRMS.10 Both Phase III studies were randomised, double-blind, placebo controlled, multi-centre international trials and enrolled over 1200 patients each. They both contained two active arms using DMF at 240mg twice a day (BID) and 240mg three times a day (TID) compared to a placebo arm randomised on a 1:1:1 ratio. CONFIRM contained an additional open label comparator arm of GA, however the study was not powered to demonstrate superiority of DMF to glatiramer acetate (GA) but just compare GA to placebo. Only the results from the DMF BID (treatment dose) will be reviewed here (Table 1).
The primary outcome of DEFINE was the proportion of patients who had relapsed at the end of the study at two years.7 The result was a relative risk reduction in relapse of 49% for the DMF arm compared to placebo (p<0.0001). Secondary endpoints were the more familiar annualised relapse rate (ARR) and rate of confirmed disability progression. Both were reduced in the DMF arm; ARR by 53% (0.172 versus 0.364 respectively, p<0.001) and the rate of confirmed disability progression by 38% (p=0.005). A subset of 540 (44%) of patients formed the MRI study, the outcomes are also summarised in Table 1.
The second phase III study pretty much did what it said on the tin. CONFIRM found ARR (the primary endpoint) was significantly reduced by 44% for DMF compared to placebo (0.224 versus 0.401 resepctively, p<0.001).8 Although not directly comparable to DEFINE a secondary endpoint of CONFIRM was proportion of patients who relapsed at two years which showed a 34% relative risk reduction compared to placebo (p=0.002). Disappointingly the confirmed disability progression endpoint was not significant. As in other studies that have failed to show a significant effect on accumulation of disability it has been suggested the failure of significance is due to lack of change in the placebo arm however this can only be supposition. CONFIRM contained a sub group of 681 patients who participated in the MRI sub-study, the results are again shown in Table 1. The open label comparator arm of GA was useful as an anchor demonstrating the expected 29% reduction in ARR compared to placebo (0.286 versus 0.401, p=0.0128), suggesting the DMF results are what would be seen in the real world.
Safety
In both DEFINE and CONFIRM there were no significant differences in serious adverse events and all adverse events between the active and placebo arms, including the GA group.7-8 The events that occurred more frequently in the DMF groups were flushing with around 40% versus 6% in placebo, and any gastrointestinal events 50% versus 40% in placebo.7-8 The number of patients that discontinued treatment due to flushing was around 3% and for gastrointestinal events 4% across studies.7-8 Importantly these side effects were most marked in the first month and then declined. They can also be reduced by taking DMF with food, or taking acetylsalicylic acid prior to taking DMF has been shown to be beneficial over a short period.11 In the current Summary of Product Characteristics (SmPc) for Tecfidera it is also possible to reduce the dose to 120mg DMF BID for up to four weeks, to allow the side effects to settle.12
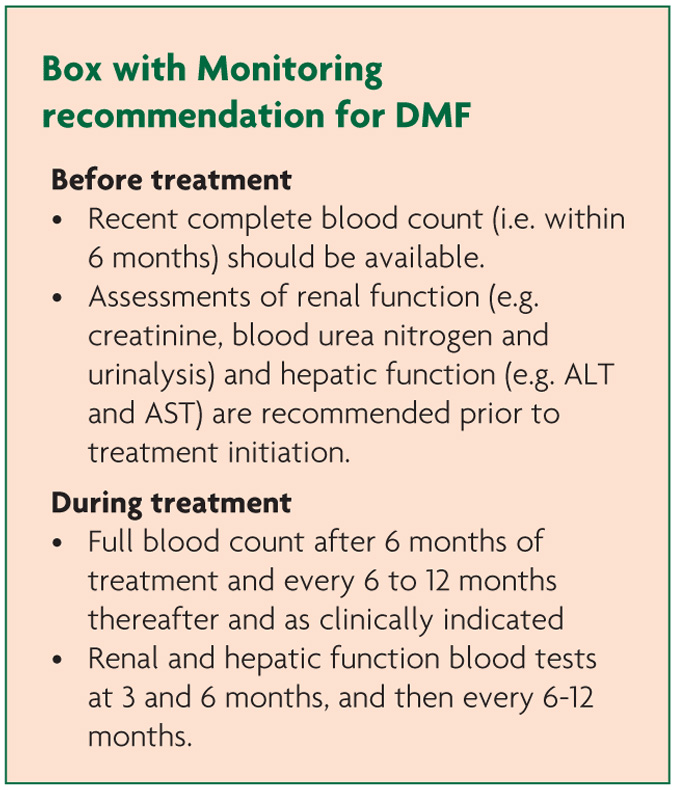
In both studies lymphocyte counts were reduced by 30% on average with 6% of patients having levels <0.5×109/L.7-8 Importantly there was no increase in serious infections in this group. Indeed there was no significant difference in the incidence of infections across the study arms. Interestingly dermatologists do use lymphocyte counts as a marker of treatment effect with Fumaderm.13
Elevations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≥3 times the upper limit of normal (ULN) were seen, respectively, in 5% and 2% of patients treated with placebo and in 6% and 2% of patients treated with DMF. These were all transient or reversed on drug withdrawal and none led to hyperbilirubinaemia or hepatic toxicity. Discontinuation due to raised transaminases was similar between DMF and placebo at <1%.
Proteinuria occurred in approximately 9% of those taking DMF but this was not dissimilar to the placebo group, and there was no significant difference in all renal events. The MS study has not revealed nephropathy or renal impairment and psoriasis studies have only noted clinically insignificant haematuria and leucocyturia that was present similarly between treatment and placebo groups.2
In the pivotal studies discussed here there was no indication of increased malignancy. ENDORSE has reported 14 cases of malignancies of differing types and locations in 13 patients.9
There is no evidence from animal studies to suggest DMF is associated with reduced fertility.14 Animal studies have shown reproductive toxicity, so DMF is not recommended during pregnancy and in women of childbearing potential not using appropriate contraception.12 Obviously with the pregnancy registry a picture of risk will develop.
Finally looking at CONFIRM the number of patients who withdrew from treatment was similar across placebo (36%), DMF BID (30%), and GA (25%). For DEFINE the figures were placebo (35%) and DMF BID (31%). In DEFINE there was a death in each of the DMF arms, both from accidents. One death occurred in CONFIRM in the TID arm from a MS relapse.
On going to press there has been a fatal case of progressive multifocal leukoencephalopathy (PML) in a patient treated with DMF for four and a half years, they had been on placebo for 2 years in a pivotal trial and entered the open label phase. It appears the crucial feature was the patient was lymphopenic throughout the treatment phase, therefore immunocompromised and predisposed to PML.
References
- National Institude of Clinical Excellence. Multiple sclerosis (relapsing-remitting) – dimethyl fumarate: evaluation report. 2014. http://guidance.nice.org.uk/TAG/340/Consultation/EvaluationReport/pdf/English.
- Mrowietz U, Christophers E, Altmeyer P. Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use. The German Fumaric Acid Ester Consensus Conference. Br J Dermatol 1999;141:424-9.
- Scannevin RH, Chollate S, Jung MY, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like pathway. J Pharmacol Exp Ther 2012;341:274-84.
- Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134:678-92.
- Litjens NH, Burggraaf J, van Strijen E, et al. Pharmacokinetics of oral fumarates in healthy subjects. Br J Clin Pharmacol 2004;58:429-32.
- Ruggieri S, Tortorella C, Gasperini C. Pharmacology and clinical efficacy of dimethyl fumarate (BG-12) for treatment of relapsing–remitting multiple sclerosis. Ther Clin Risk Manag 2014;10:229-39.
- Gold R, Kappos L, Arnold DL, et al; DEFINE Study Investigators. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367(12):1098-107.
- Fox RJ, Miller DH, Phillips JT, et al; CONFIRM Study Investigators. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087-97.
- Phillips JT, Fox R, Selmaj K, et al. Safety and tolerability of oral BG-12 (dimethyl fumarate) in relapsing–remitting multiple sclerosis (RRMS): interim results from ENDORSE Extension Study (P01.162). Neurology. 2013;80(Meeting Abstracts 1):P01.162.
- Kappos L, Gold R, Miller DH, et al; BG-12 Phase IIb Study Investigators. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo controlled phase IIb study. Lancet. 2008;372:1463-72.
- O’Gorman J, Russell H, Li J, et al. Effect of Aspirin pretreatment or slow dose titration on the incidence and severity of flushing and gastrointestinal events associated with BG-12 (dimethyl fumarate). Poster presented at: ECTRIMS Poster Session 1, “Treatment of Specific Symptoms”; October 3, 2013; Copenhagen, Denmark.
- Tecfidera™ (dimethyl fumarate). Summary of product characteristics. Biogen Idec Ltd, UK, 2014.
- Ralf Gold. Consulant Neurologist. Personal Communication. Day abbreviated month year.
- Gold R, Phillips T, Haavrdova E, et al. BG-12 (Dimethyl Fumarate) and Pregnancy: Preclinical and Clinical Data from the Clinical Development Program. Neurology 2013;80(Meeting Abstracts 1):P02.129.


