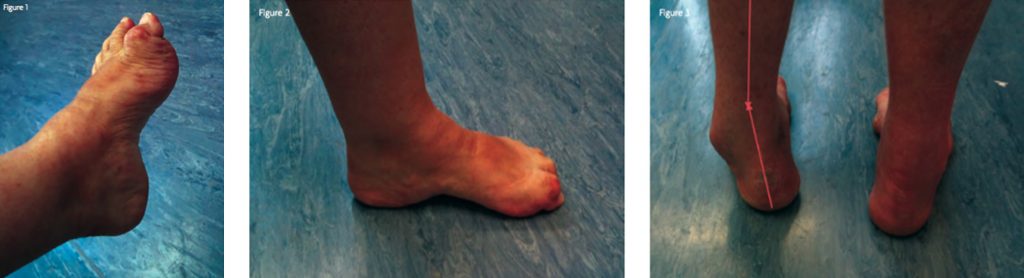
The term Pes cavus describes the deformity of a high arched, relatively stiff foot. It has a variety of neurological and other causes. Management depends on the aetiology, rapidity of progression and the severity of symptoms. Definition Pes cavus is an umbrella term describing a spectrum of foot shapes with a high arch [1]. Pure Pes cavus occurs when the metatarsal bones are plantarflexed relative to the hindfoot – described as ‘forefoot plantaris’ – which increases the height and curvature of the medial longitudinal arch (Figure 1). When the patient weight-bears, the hindfoot is pushed into dorsiflexion by the plantarflexed forefoot (Figure 2).
A high arch accompanied by a medially angulated heel is termed pes cavovarus (Figure 3). When this is complicated by foot drop and equinus of the ankle, it is described as pes equinocavovarus. Another variant, pes calcaneovarus, occurs when the primary deformity is excessive ankle and hindfoot dorsiflexion; in order to place the foot flat on the ground, the forefoot plantarflexes, leading to a high arch.
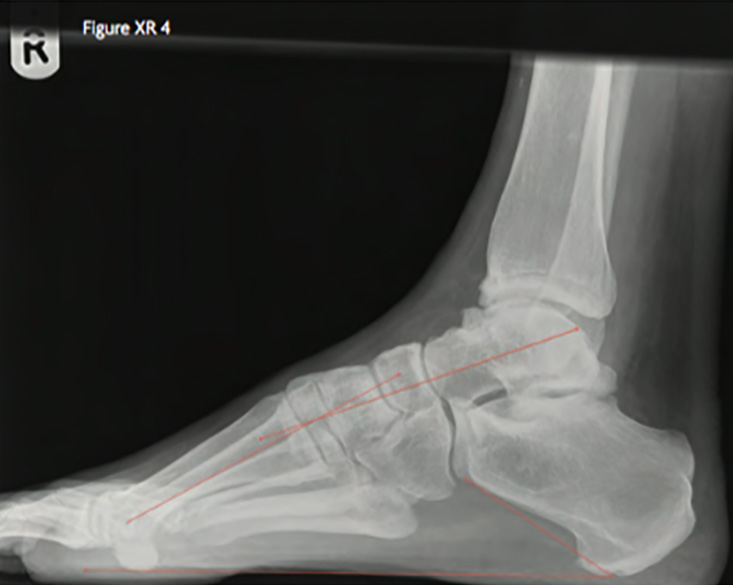
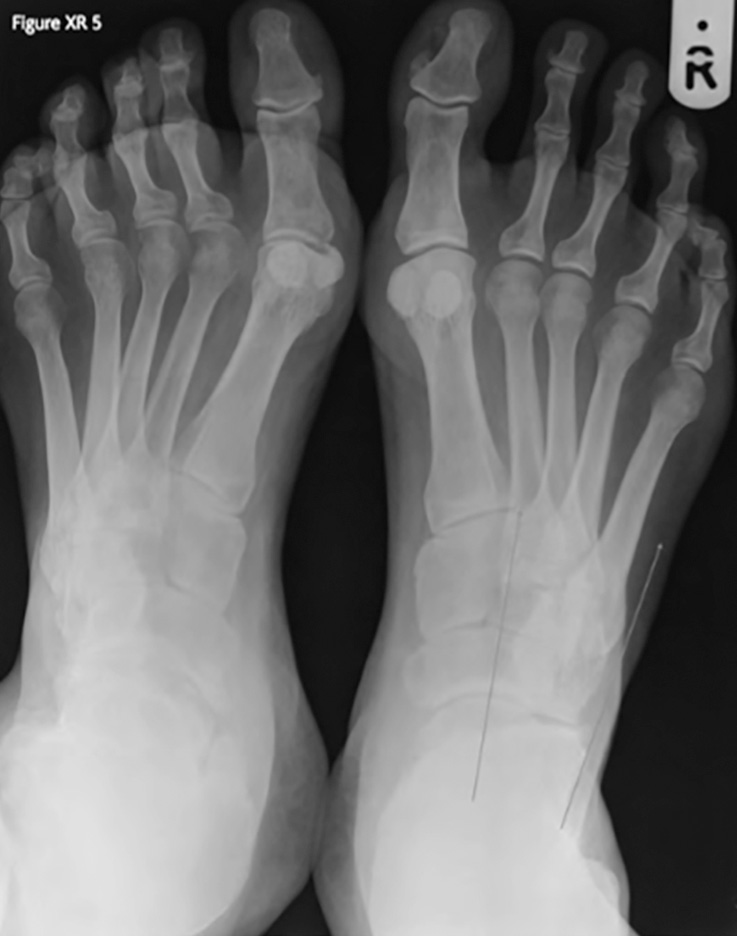
On radiographs, a high arch manifests as a Meary’s angle of over 5 degrees – the angle between the long axis of the talus and the first metatarsal in the lateral view (Figure XR 4). The talus and the calcaneum are dorsiflexed, with calcaneal pitch exceeding 30 degrees [1]. The calcaneum appears shortened when in varus. On the dorso-plantar view, supination is seen as a narrow talo-calcaneal angle (Figure XR 5).
The wide spectrum of normality leads to controversy over the inclusion of milder variants in the definition of pes cavus. An objective measure of the degree of supination or pronation, the Foot Posture Index (FPI), has been developed and validated [2]. However, while the FPI describes and quantifies foot shape, it does not delineate the normal foot from pes cavus. In practice, what is important is that subtle cases of pes cavus are identified and that potential pathology is considered.
Aetiology, classification and pathophysiology
A list of causes is given in box 1. These conditions have differing pathophysiology, but unbalanced muscular forces are almost always at the root of caves feet.
The sole of the foot can be conceived as a tripod, consisting of the first metatarsal head, fifth metatarsal head and heel. All three points should be in contact with the ground during stance, with the ankle balanced over the triangular base that they form. In typical cases of pes cavovarus associated with HSMN [3], tibialis posterior and peroneus longus are consistently stronger than tibialis anterior (TA) and peroneus brevis [6], resulting in plantarflexion of the first ray and adduction of the forefoot [4,7]. This creates pronation of the forefoot relative to the hindfoot [8]. The plantar fascia contracts, further depressing the first metatarsal head. To keep the tripod flat on the ground, the hindfoot dorsiflexes and supinates into varus.
Hindfoot varus may be flexible at first but becomes progressively fixed [1,8]. Once the heel’s point of contact is medial to the centre of the ankle, there is a moment that tends to exacerbate the hindfoot varus. The Achilles tendon’s insertion is medialised and it becomes a secondary inverter [1]. Thus pes cavovarus may start as a forefoot-driven phenomenon and later become hindfoot-driven.
Frequent toe deformities are partly caused by weak intrinsic foot muscles. They are overcome by the extrinsic muscles: extensor digitorum longus (EDL), flexor digitorum longus (FDL), extensor hallucis longus (EHL) and flexor hallucis longus (FHL). The imbalance leads to hyperextension of the metatarso-phalangeal joints (MTPJs) and flexion of the interphalangeal joints, or ‘clawing’. This is made worse when EHL and EDL are recruited to aid a weak TA in dorsiflexing the foot [4]. The plantar fascia, attached to the proximal phalanges, tightens when they hyperextend. This exaggerates the medial longitudinal arch via the windlass mechanism [1]. Eventually, the slips of plantar fascia toes become dorsal to the centre of the MTPJ, thus becoming a perverse extensor. A theory that weak intrinsic muscles account for all the deformities of pes cavus [9] cannot, however, be sustained [10,6].
The Equino-cavovarus deformity is seen as the end stage of HSMN-induced pes cavus. Once there is no active dorsiflexion, the ankle plantarflexes, the calf muscles and posterior joint capsule contract, and equinus ensues.
The calcaneovarus variant is seen in poliomyelitis, low spinal dysraphism, or after overzealous surgical lengthening of the Achilles tendon. Paralysis of the calf muscles leads to excessive ankle dorsiflexion and compensatory forefoot plantaris.
In children, the skeleton reacts to the forces upon it, with differential growth at the physis, according to Wolff’s law. The muscle imbalances cause deformities that initially occurred through the joints to become fixed in the bony architecture of the mature foot.
Box 1: Causes of Pes cavus
Progressive neurological disorders
• Hereditary Sensorimotor Neuropathies (HSMN) or Charcot-Marie-Tooth disease (CMT) (78%) [3]
• Hereditary sensory and autonomic neuropathies
• Friedreich ataxia
• Spinal or brain Tumour
• Spinal muscular atrophy
• Spinal trauma
• Syringomyelia
• Myelodysplasia
• Spinal dysraphism: spina bifida, spina bifida occulta, diastematomyelia4
• Muscular dystrophy
Static neurological disorders
• Cerebral palsy
• Stroke
• Poliomyelitis
• Spinal nerve root injury
• Peroneal nerve injury [4]
Other causes
• Scarring of the deep posterior compartment after compartment syndrome5
• Foot trauma
• Tarsal coalition
• Under-corrected congenital talipes equinovarus
• Iatrogenic (e.g. overzealous surgery for pes planus)
• Idiopathic / familial
Epidemiology and Genetics
Some authors quote the prevalence of high-arched feet as 8-15% in the population [11,12]; but the true prevalence of pes cavus associated with pathology is much lower. About 37 in 100,000 people are affected by CMT [7]. A recent review has summarised our knowledge of the varied phenotypes and their genetic basis [13]. In the UK, 90% of patients have autosomal CMT type 1 (demyelinating) or type 2 (axonal). Most mutations involve the peripheral myelin protein 22 gene on chromosome 17. Genetic testing has progressed such that 70% of patients can be given a genetic diagnosis. Our ability to relate the different forms of these genes to their variable phenotypic expression is still limited, although mRNA levels of some lipid metabolism genes in skin biopsies may offer a way to predict phenotypic expression [13]. CMT type 2 seems to produce milder symptoms with a slower progression than type 1 [7].
Symptoms and signs, and their relationship to biomechanics
The foot serves as an organ of load distribution, shock absorption, balance and propulsion. Pes cavus interferes with all of these functions [1]. Supination of the hindfoot normally results in a change of the foot from a loosely packed, flexible, energy-absorbing structure to a tightly packed, stiffer lever. This change occurs naturally during the gait cycle. When the hindfoot remains supinated throughout the gait cycle, however, the reduced flexibility lessens the foot’s capability as a shock absorber [1] and diminishes its ability to balance on uneven ground. Hindfoot varus also leads to an increased moment on the ankle [11], making ankle inversion injuries common [14]. Eventually there may be dramatic varus tilting of the ankle and secondary osteoarthritis [15].
Forefoot plantaris leads to increased pressure on the metatarsal heads. This pressure is maintained for a greater proportion of the gait cycle than in normal feet [16,17]. A high arch reduces the size of the footprint and increases plantar pressure. Plantar pain and callus formation may give way to ulceration, particularly in the neuropathic patient who lacks protective sensation.
Neuropathies may be accompanied by neuropathic pain. It is essential that mechanical symptoms, which can be treated by orthoses or surgery, are distinguished from neuropathic pain, which cannot.
With progression, deformity and rigidity become more severe. This leads to overload of the lateral side of the foot and stress fractures of the fifth metatarsal [14]. Peroneal tendinopathy, Achilles tendon disorders, plantar fasciitis, and ankle impingement are also more common in the cavus foot [5].
Box 2: Symptoms of pes cavus
• Metatarsal overload
• Heel pain
• Lateral overload
• Stiffness
• Ankle sprains
• Ill-fitting footwear, toes rubbing
• Ulceration
Clinical evaluation & investigation
It is critical to establish whether there is a neurological diagnosis and whether it is progressive or static [18]. In the growing foot, the deformity may be progressive although the neurological impairment may be static.
The history should cover the onset of foot problems and how they have progressed. Pain, instability, difficulty walking or running and problems with footwear are frequent complaints. Neurological symptoms, such as sensory changes, weakness and clumsiness should be sought. Back pain or headaches may signify a central cause. Family history may suggest a hereditary cause.
General examination may reveal features of neurological conditions such as “champagne bottle legs” (Charcot-Marie-Tooth disease), scoliosis in Friedreich ataxia, or a naevus, dimple or patch of hair over the spine in spina bifida occulta. The neurological examination should include a search for signs of peripheral nervous disease, such as muscle wasting, weakness and sensory deficit, and signs of central nervous disease, such as pyramidal signs, cerebellar signs or cranial nerve abnormalities. Accurate serial recording of power in individual muscle groups will allow the clinician to follow the disease over time and detect neurological progression.
The key in examining the foot is to determine to what extent deformities are fixed or flexible. This guides orthotic and surgical treatment. Gait is inspected; in HSMN the typical gait is high-stepping because of foot-drop, with the toe striking the ground before or with the heel [17]. Foot shape is best assessed with the patient standing [4,5]. The soles are inspected for calluses and the shoes for differential wear (indicating sites of excessive pressure). Tender areas, such as the metatarsal heads or base of the fifth metatarsal, are palpated. Passive movements should be assessed, looking for joint contractures. Testing active movements detects muscle weakness. The Coleman block test [8] is one way to determine whether the hindfoot is flexible. With the patient standing, the heel and fifth ray are placed on a wooden block, permitting the forefoot to pronate. If the hindfoot also pronates, it is flexible; if not, it is in fixed varus.
Investigations will be guided by the suspected aetiology. Weight-bearing radiographs are performed to assess the degree of bony deformity and look for arthritic changes. In cases of suspected HSMN, nerve conduction testing and electromyography may be useful. A CMT DNA duplication detection test may be performed for confirmation. If the onset is during adulthood, and especially if rapidly progressive or unilateral, a central disorder such as spinal dysraphism or a space-occupying lesion must be excluded by magnetic resonance imaging of the brain and spinal cord.
Treatment
Non-surgical treatments
Non-surgical treatment is instituted early and is chiefly delivered by podiatrists and orthotists, preferably working alongside doctors in a foot and ankle clinic. Orthotic treatment can broadly be separated into four types: pressure relief, correction of deformity, accommodation of deformity, and splinting. Chiropodists and podiatrists can provide simple devices, but more involved orthoses are made by an orthotist. A simple cushioning orthosis alone may help symptoms from pressure overload [12]. Pressure on the metatarsal heads is alleviated by a total contact orthosis that widens the contact area [11,19]. One randomised controlled trial [12] has compared custom-molded, semi-rigid orthoses with soft, sham inserts. The custom inserts caused a clinically and statistically significant reduction in foot pain scores and peak plantar pressure at three months, and a significant increase in quality of life measures.
If a considerable part of the deformity is flexible, a corrective orthosis should be used. For example, in forefoot-driven cavovarus, the hindfoot is flexible, and so an orthotic shoe insert incorporating lateral forefoot posting (support) and recessing under the first metatarsal will allow the hindfoot to correct [5]. For ankle instability, the lateral side of the hindfoot post can be built up as well as the lateral forefoot post [11], creating a pronatory moment on the forefoot that counteracts the excessive supinatory moment in the hindfoot.
Any fixed deformity must be accommodated, for example by cupping and supporting the varus heel and providing a small heel raise to compensate for forefoot plantaris. It has been shown that an orthosis that allows the first metatarsal to drop can decrease calcaneal dorsiflexion, and that this coincides with a reduction in foot pain [20].
With increasing paralysis, splints or appliances are used. If there is a degree of flexibility in the deformity, semi-rigid custom-made ankle-foot orthoses (AFO) can both correct and stabilise the foot and ankle, but can be worn inside a normal shoe and are thus preferred by many patients. If swelling or a fixed deformity cause rubbing and pressure in an AFO, custom-made boots are an alternative. For very severe deformity and refractory ankle instability, in a patient who cannot tolerate an AFO, a caliper incorporating an inside iron and outside T strap may be prescribed.
Physiotherapy is often directed at maximising muscle power [21] and stretching stiff joints. A typical program would aim at strengthening dorsiflexors and everters (EHL, TA, peroneus brevis) and stretching inverters (tibialis posterior). A review of the two published randomised controlled trials of night time ankle bracing found that it had no effect on ankle flexibility in patients with CMT1A [22]. Thus, in the face of a pronounced or progressive neurological deficit, efforts are probably best directed towards providing aids that optimise function.
Botulinum toxin type A injections into peroneus longus and tibialis posterior have been trialled in patients with CMT1A without success [23]. If non-invasive treatment alleviates symptoms and can delay progression, it is clearly preferable to surgery. However, there are no longitudinal studies showing that conservative treatments alter the course of pes cavus [7].
Surgical treatment
Surgery is a last resort if the above conservative measures fail to control symptoms. Surgery is only justified when the deformity is so pronounced or progressive that symptoms are intrusive and unresponsive to conservative treatments. On the other hand, surgery should not be delayed so long that severe ulceration develops or the patient cannot ambulate. A timely, limited surgical intervention, while the foot is still flexible, can rebalance the foot and prevent the need for a larger, more technically demanding procedure later on [15].
The aims of surgery are threefold:
- To correct deformity, thereby placing a balanced, stable, plantigrade foot on the ground with even plantar pressures between heel, first ray and fifth ray.
- To relieve pain due to overloaded or arthritic joints, while preserving joint motion where possible.
- To re-balance muscle forces, aiding in gait and preventing progression or recurrence of deformity.
In principle, these aims are achieved by means of [15]:
- Joint releases and tendon lengthening.
- Tendon transfers, taking over-powerful, mechanically advantaged tendons and transferring them to weaker, disadvantaged tendons.
- Osteotomies: dividing and re-aligning bones, and stabilising with plaster or internal fixation.
- Arthrodeses: fusing stiff, painful joints
A critical element of any attempted surgery is re-balancing the forces across the foot. A common example is the Bridle procedure, splitting tibialis posterior and transferring half to tibialis anterior and half to the lateral side of the dorsum of the foot. Peroneus longus is commonly transferred to augment peroneus brevis [4]. Soft tissue procedures are occasionally performed in isolation in the paediatric foot.
When there is limited deformity and rigidity, osteotomies are preferred to arthrodesis if possible, as they preserve motion. The first metatarsal is often treated with a dorsal closing wedge osteotomy, and the heel is lateralised with a sliding osteotomy [24]. Even after a good correction with well-healed osteotomies, neurological progression may cause recurrent deformity, typically five to ten years later [15], necessitating arthrodesis [18].
Toe deformities can be effectively treated with the Jones [25] and Hibbs procedures. These correct the cock-up deformities by fusion of the interphalangeal (IP) joints, combined with transfer of the EHL and EDL tendons to the metatarsal necks to assist with ankle dorsiflexion [4]. The EHL and EDL transfers remove the deforming force on the MTP joint, and relax the plantar fascia.
In patients with inflexibility, arthrodesis sacrifices little, and relieves joint pain. The foot can be re-orientated by excising wedge-shaped portions of the joints. The triple arthrodesis of subtalar, talonavicular and calcaneocuboid joints is very commonly used [24,15]. Midfoot arthrodeses may be more appropriate, depending on the maximum site of pain and deformity.
Finally, the arthritic ankle may require arthrodesis or replacement. However, this is doomed to failure unless the foot position is corrected and the muscles re-balanced at the same time [15].
While these general principles have evolved from collective experience and discussions between expert commentators, there is a distinct lack of good quality level I and level II evidence comparing different treatments [7]. In this relatively rare disorder with a heterogeneous presentation, treated by various surgeons in different ways, randomised controlled trials are for the moment elusive. Summary Pes cavus is a complex deformity, which can arise from neurological aetiology. Despite centuries of experience, controversies still exist as to its precise definition, pathophysiology, prognosis and treatment. The clinician should always identify the cause, whether it is progressive or static, and address the symptoms, tailoring conservative treatment according to the degree of flexibility. The objective of surgical treatment is a plantigrade, stable, flexible foot, in order to improve mechanical symptoms. Neuropathic pain needs to be distinguished from mechanical pain in order to avoid inappropriate surgical interventions.
References
- Aminian A, Sangeorzan BJ. The anatomy of cavus foot deformity. Foot Ankle Clin 2008;13:191-8, v. https://doi.org/10.1016/j.fcl.2008.01.004
- Redmond AC, Crosbie J, Ouvrier RA. Development and validation of a novel rating system for scoring standing foot posture: the Foot Posture Index. Clin Biomech (Bristol, Avon) 2006;21:89-98. https://doi.org/10.1016/j.clinbiomech.2005.08.002
- Nagai MK CG, Guille JT et al. Prevalence of Charcot-Marie-Tooth disease in patients who have bilateral cavovarus feet. J Pediatr Orthop 2006;26:438-43. https://doi.org/10.1097/01.bpo.0000226278.16449.c4
- Marks RM. Midfoot and forefoot issues cavovarus foot: assessment and treatment issues. Foot Ankle Clin 2008;13:229-41, vi. https://doi.org/10.1016/j.fcl.2008.02.007
- Chilvers M, Manoli An. The subtle cavus foot and association with ankle instability and lateral foot overload. Foot Ankle Clin 2008;13:315-324. https://doi.org/10.1016/j.fcl.2008.01.003
- Burns J, Redmond A, Ouvrier R et al. Quantification of muscle strength and imbalance in neurogenic pes cavus, compared to health controls, using hand-held dynamometry. Foot Ankle Int 2005;26:540-544. https://doi.org/10.1177/107110070502600708
- Beals TC, Nickisch F. Charcot-Marie-Tooth disease and the cavovarus foot. Foot Ankle Clin 2008;13:259-74, vi-vii. https://doi.org/10.1016/j.fcl.2008.02.004
- Coleman SS, Chesnut WJ. A simple test for hindfoot flexibility in the cavovarus foot. Clin Orthop Relat Res 1977;60-62. https://doi.org/10.1097/00003086-197703000-00022
- Gallardo E, Garcia A, Combarros O et al. Charcot-Marie-Tooth disease type 1A duplication: spectrum of clinical and magnetic resonance imaging features in leg and foot muscles. Brain 2006;129:426-437. https://doi.org/10.1093/brain/awh693
- Burns J, Ouvrier R. Pes cavus pathogenesis in Charcot-Marie-Tooth disease type 1A. Brain 2006;129:E50; author reply E51. https://doi.org/10.1093/brain/awl116
- Scherer PR. Orthotic management of the pes cavus foot. Lower Extremity Review Magazine 2011
- Burns J, Crosbie J, Ouvrier R et al. Effective orthotic therapy for the painful cavus foot: a randomized controlled trial. J Am Podiatr Med Assoc 2006;96:205-211. https://doi.org/10.7547/0960205
- Pandraud A LY, Houlden H. of Peripheral Nerve Advances in the genetics of peripheral nerve Disorders. ACNR 2012;12:8-13.
- Williams DSr, McClay IS, Hamill J. Arch structure and injury patterns in runners. Clin Biomech (Bristol, Avon) 2001;16:341-347. https://doi.org/10.1016/S0268-0033(01)00005-5
- Sraj SA, Saghieh S, Abdulmassih S et al. Medium to long-term follow-up following correction of pes cavus deformity. J Foot Ankle Surg 2008;47:527-532. https://doi.org/10.1053/j.jfas.2008.06.007
- Burns J, Crosbie J, Hunt A et al. The effect of pes cavus on foot pain and plantar pressure. Clin Biomech (Bristol, Avon) 2005;20:877-882. https://doi.org/10.1016/j.clinbiomech.2005.03.006
- Crosbie J, Burns J, Ouvrier RA. Pressure characteristics in painful pes cavus feet resulting from Charcot-Marie-Tooth disease. Gait Posture 2008;28:545-551. https://doi.org/10.1016/j.gaitpost.2008.03.011
- McCluskey WP, Lovell WW, Cummings RJ. The cavovarus foot deformity. Etiology and management. Clin Orthop Relat Res 1989;27-37. https://doi.org/10.1097/00003086-198910000-00006
- Benedetti MG CF, Ceccarelli F et al. Gait analysis in pes cavus. Gait Posture 1997;5:169.
https://doi.org/10.1016/S0966-6362(97)83405-4 - Eslami M, Tanaka C, Hinse S et al. Acute effect of orthoses on foot orientation and perceived comfort in individuals with pes cavus during standing. Foot (Edinb) 2009;19:1-6. https://doi.org/10.1016/j.foot.2008.06.004
- Sackley C DPB, Turner-Stokes L et al. Rehabilitation interventions for foot drop in neuromuscular disease. Cochrane Database Syst Rev 2007;18:CD003908. https://doi.org/10.1002/14651858.CD003908.pub2
- Rose KJ, Burns J, Wheeler DM et al. Interventions for increasing ankle range of motion in patients with neuromuscular disease. Cochrane Database Syst Rev 2010;CD006973. https://doi.org/10.1002/14651858.CD006973.pub2
- Burns J, Scheinberg A, Ryan MM et al. Randomized trial of botulinum toxin to prevent pes cavus progression in pediatric Charcot-Marie-Tooth disease type 1A. Muscle Nerve 2010;42:262-267. https://doi.org/10.1002/mus.21685
- Klaue K. Hindfoot issues in the treatment of the cavovarus foot. Foot Ankle Clin 2008;13:221-7, vi. https://doi.org/10.1016/j.fcl.2008.02.005
- Tynan MC, Klenerman L. The modified Robert Jones tendon transfer in cases of pes cavus and clawed hallux. Foot Ankle Int 1994;15:68-71. https://doi.org/10.1177/107110079401500203


