Abstract
Adults with intellectual disability are more likely to experience sleep disorders and disordered sleep, which not only adversely affects their mental and physical health, but also the health and wellbeing of their carers. Despite this, there are neither recommended assessment nor treatment guidelines for this population. In this brief narrative review, we summarise what is known about diagnosing and managing sleep disorders in adults with intellectual disability, share our clinical experience, and highlight the need for further research.
Introduction
Sleep disorders are more common in adults with intellectual disability (ID), with higher prevalence rates (up to 100%) when compared to those in the general population [1]. Despite this, the importance of sleep in ID is often overlooked, especially clinically, where sleep disorder(s) may be viewed as being part of the disability (i.e. diagnostic overshadowing), and/or too challenging to investigate and treat [2-4]. In addition to adversely affecting physical and mental health, sleep disorders in adults with ID may also drive challenging behaviours, increase carer burden, and as a result, increase health and social care utilisation [5-7].
Despite the increased prevalence and adverse sequelae of sleep disorders in adults with ID, there is a paucity of research and guidance regarding optimal assessment, diagnostic and management approaches [2,8-11]. In this brief narrative review, we discuss the current evidence base, as well as our experience, in assessing, diagnosing, and managing common sleep disorders in this population.
Epidemiology
Adults with intellectual disabilities represent a special population with notable health inequalities, such as increased multi-morbidity, and up to an 18-year reduced life expectancy [12]. Both sleep disorders and disordered sleep are very common in adults with ID, and as for the general population, represent a potentially important modifiable health risk factor [13,14]. Owing to the heterogeneity and quality of epidemiological studies in adults with ID, prevalence rates of disordered sleep vary widely. A recent systematic review of twenty studies (> 8, 000 participants) identified prevalence rates of disordered sleep (across a range of sleep parameters) ranging from 6.1 to 74.2%, whilst studies examining sleep-related breathing disorders (> 2,500 participants) identified prevalence rates ranging from 0.5 to 100% [1].
Assessment
We would encourage any clinician involved in the care of an adult with ID to inquire about their sleep. Whilst this may sound simplistic, it is frequently overlooked e.g. sleep is not included in the NHS Learning Disabilities Annual Health Check [15].
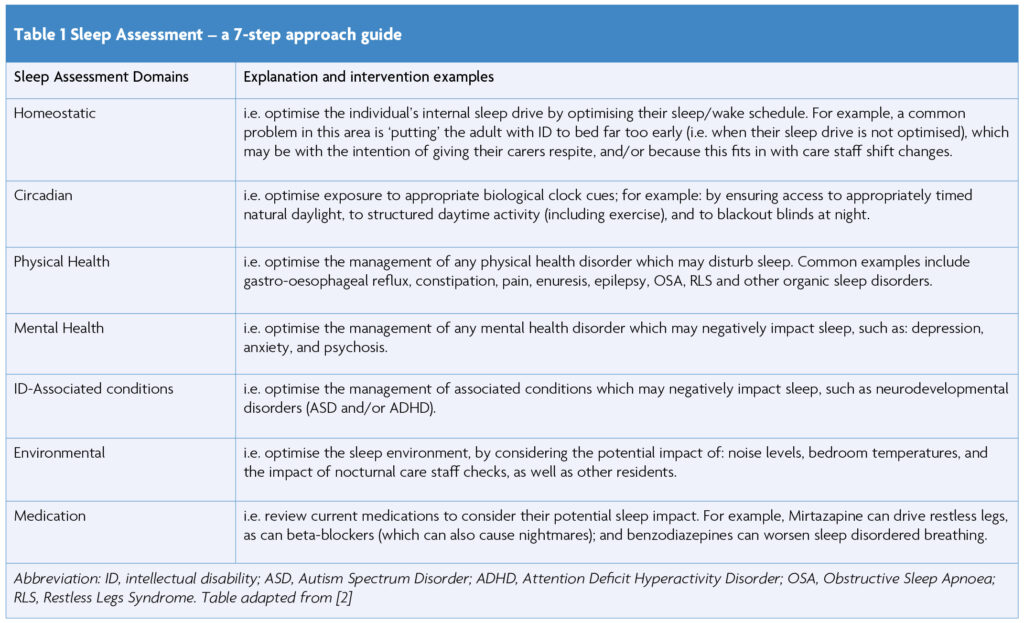
When an individual and/or their carer identifies a sleep concern, adopting a person-centred approach can help elucidate the biological, psychological, behavioural, and social factors which may be implicated. For example, in our clinic, we use a 7-step approach to help guide our assessment (Table 1).
Adults with ID are a heterogenous group, and so it is important to bear in mind that specific sleep disorders can cluster around genetic syndromes or disorders, as well as comorbid neurodevelopmental disorders [2,15]. For example, adults with Down’s Syndrome have an increased risk of Obstructive Sleep Apnoea (OSA), likely due to the characteristic features of this syndrome e.g. cranio-facial abnormalities, hypotonia, and obesity (as a result, it is recommended that everyone with Down’s Syndrome is screened for OSA.); whilst adults with Smith-Magenis Syndrome have a higher risk of circadian sleep-wake disorders, classically presenting with an inversion of their sleep-wake cycle, secondary to melatonin dysregulation [16-19].
Diagnosis
Unlike the general population, adult ID-specific sleep diagnostic guidelines are lacking. Subjective sleep information may be more commonly provided by families/carers, who may have differing opinions on the level of sleep disturbance, or they may simply accept poor sleep as part of the person’s underlying condition [20]. As a result, sleep disorders are more likely to be reported when they lead to nocturnal and daytime dysfunction, including behavioural disturbance i.e. rarely are sleep symptoms reported because of their impact on the person’s subjective quality of life [21].
Screening questionnaires can be used to assess sleep disorder severity and monitor treatment response, but they are rarely validated in adults with ID, and again, tend to rely more on carer’s reports. A pictorial version of the Epworth Sleepiness Scale (for example) has attempted to overcome this challenge, albeit with variable success [22]; whilst the STOP-Bang questionnaire has been successfully validated for adults with Down’s syndrome who have a moderate to severe Obstructive Sleep Apnoea (OSA) [16]. The STOP-Bang questionnaire is one of the most widely accepted and easy to use OSA screening tools; it consists of four self-reportable (STOP: snoring, tiredness, observed apnoea, and high blood pressure) and four demographic (Bang: body mass index, age, neck circumference, and gender) items [23]. Individuals with a STOP-Bang score of 0 to 2 can be classified as low risk for moderate to severe OSA, whereas those with a score of 5 to 8 can be classified as high risk for moderate to severe OSA. Individuals whose STOP-Bang scores are in the midrange (i.e. 3 or 4), require further clinical evaluation to determine the likelihood of OSA [23].
Sleep diaries (usually completed by carers) and/or actigraphy (ideally worn for a minimum of two weeks) can be used to record 24-hour sleep/wake timings (helpful in diagnosing insomnia and/or circadian sleep-wake rhythm disorders for example). If a sleep diary is completed by a carer (as opposed to the individual with ID), there may be reporting errors, such as a lower estimate of wake-after-sleep-onset time (a measure of sleep maintenance) [24] .This potential confounder should be considered when undertaking sleep scheduling, where a gentler approach, such as sleep compression may be utilised [25]. Home or inpatient sleep investigations e.g. pulse oximetry, home video telemetry or inpatient polysomnography can be used to investigate for physical sleep disorders, such as sleep disordered breathing or movement disorders, and epilepsy etc. If undertaking an inpatient sleep investigation, having access to an individual room, as well as the capacity to involve family/ carers can be very helpful. Moreover, additional time may be required for equipment (e.g. electrode) acclimatisation, and sometimes creative thinking is required e.g. if an actiwatch is not tolerated, then (if clinically necessary), we often advise sewing the device into the individual’s daily clothing.
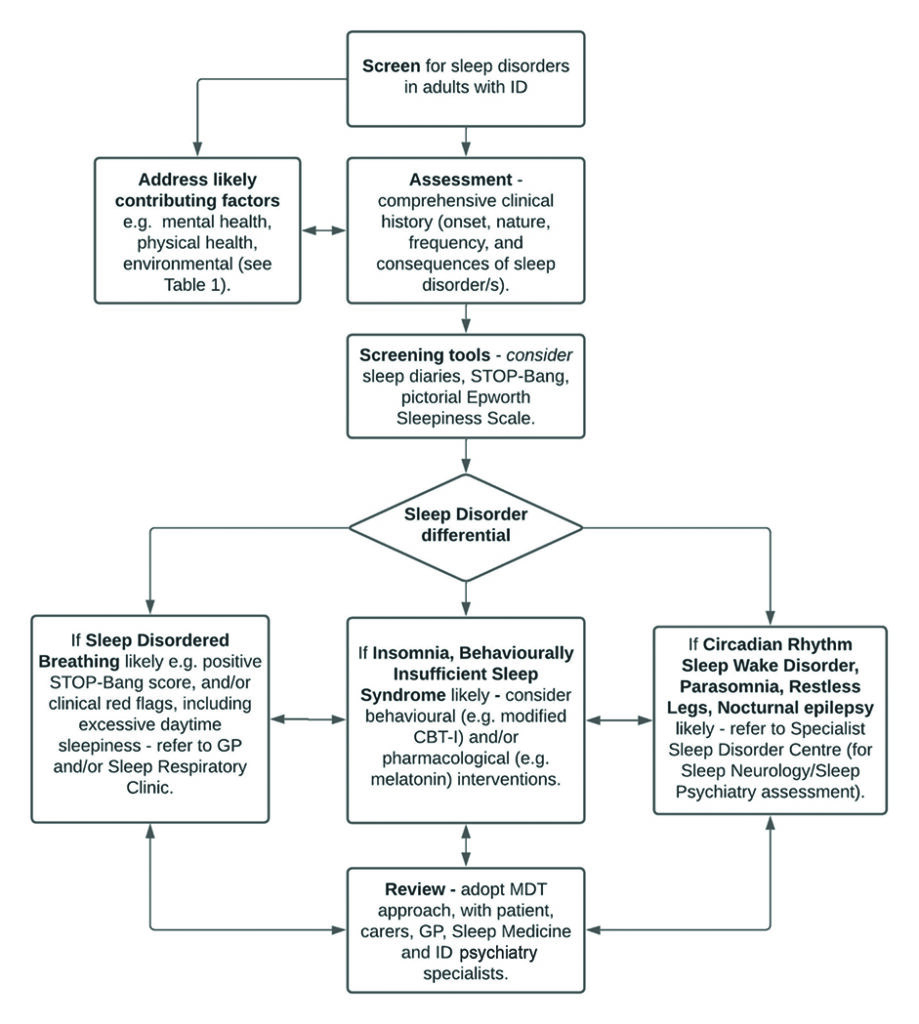
There will of course be times when an adult with ID cannot tolerate any clinically relevant sleep investigation, and in this scenario, a pragmatic trial of treatment may be required. In conjunction with intellectual disability expert colleagues, we have previously proposed one approach for the screening, assessment, and management of sleep disorders in adults with ID (Figure 1).
Management
The management of sleep disorders in adults with ID frequently requires a multi-disciplinary team (MDT) approach, and may require professional input from primary care, social care, sleep medicine and intellectual disabilities psychiatry. Such an MDT approach reflects the heterogeneity of the underlying causes of ID and their associated co-morbidities. Every attempt should be made to optimise the factors outlined in Table 1, as focusing on one area alone is unlikely to be successful (Vignette 1 & 2).
Physical treatments
Continuous positive airway pressure (CPAP) has been shown to be effective in adults with Down’s syndrome and OSA, in improving sleep, excessive daytime somnolence, mood, behavioural disturbance, general health and cognitive function [22]. Moreover, it has been demonstrated to be well-tolerated [22]. For individuals who struggle to accept CPAP, exposure therapy may be beneficial, and in this scenario, collaborative working between sleep medicine specialists and others (e.g., mental health nurses, families, carers) can be helpful. Where positive airway pressure is unsuccessful or not tolerated, consideration could be given to hypoglossal nerve stimulation [26]; and where obesity plays a maintaining role in OSA, medical management e.g. with glucagon receptor-1 agonists, could be pursued [27].
Behavioural treatments
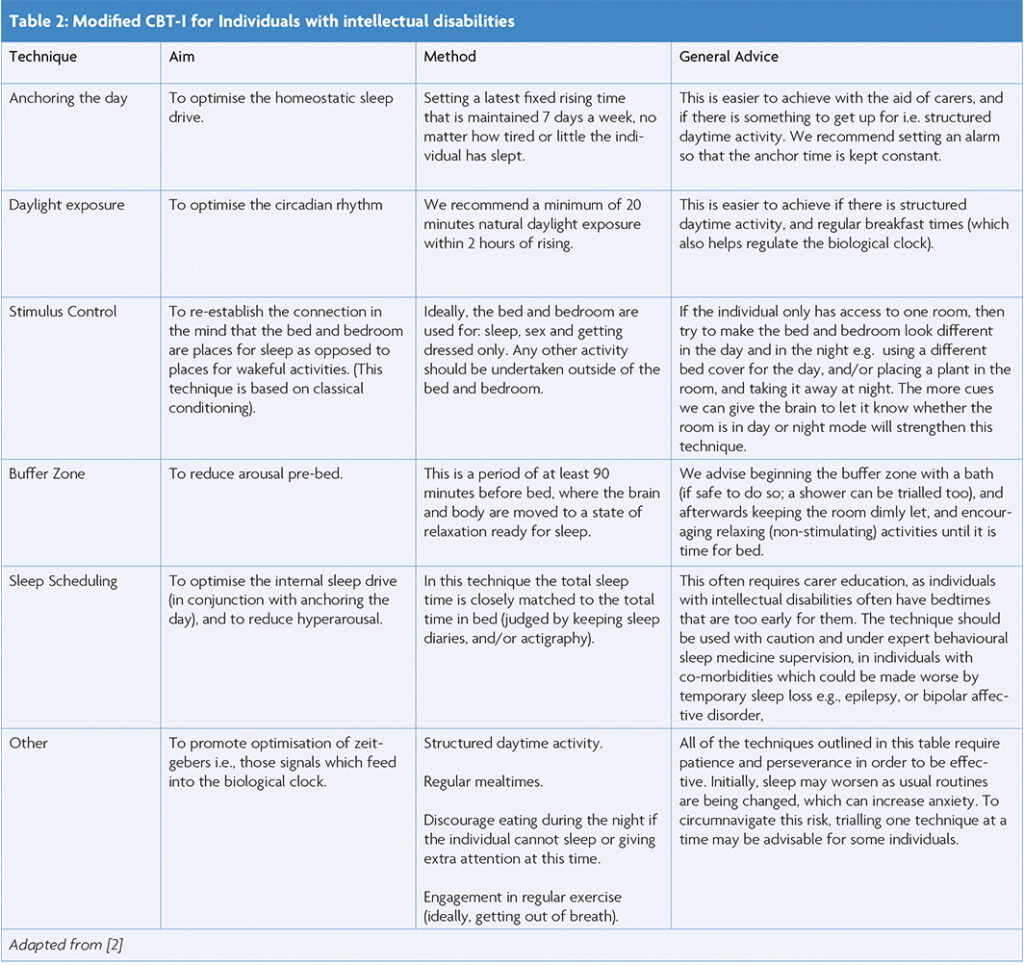
Cognitive Behavioural Therapy for Insomnia (CBT-I) is the first line recommended treatment for chronic insomnia in the general population [28], and there is some evidence that it may also be helpful in adults with ID [21]. In particular sleep scheduling has been successfully utilised, both on its own and as part of a multi-modal CBT-I treatment. In this technique the average total sleep time (obtained from sleep diaries and/or actigraphy) is closely matched to the average total time in bed. This helps to optimise the homeostatic (internal) sleep drive, reduces hyperarousal, and helps to consolidate sleep. The technique is likely to require family/carer education and flexibility, as often adults with ID have an imposed bedtime which may be too early for them [2]. In this scenario, working with social and healthcare providers to increase care package provision may be required. The technique should ideally be undertaken under the guidance of a behavioural sleep medicine specialist, as any unintended sleep deprivation may worsen both physical (e.g. epilepsy) and mental (e.g. bipolar affective) disorders [2, 29]. Additional CBT-I techniques are outlined in Table 2.
Even relatively simple modifications to scheduled daytime activities and sleeping environments can bring about significant sleep improvements e.g. adults with ID attain less daily exercise, and have an unhealthier diet when compared to the general population; two important factors which increase the risk of sleep-disordered breathing [21]. The loss of daytime colleges and placements during the Covid-19 lockdowns had (in our experience) a devastating effect on sleep in adults with ID.
Pharmacological treatments
There is again a paucity of evidence regarding the pharmacological management of sleep disorders in adults with ID, with the management of non-insomnia disorders tending to follow the same treatment algorithms as those for the general population. Unsurprisingly, the medication which has attracted most attention is melatonin, with one meta-analysis concluding that it reduces sleep onset latency, and improves sleep maintenance and total sleep time in adults with ID [30]. Melatonin is associated with few adverse events, and is relatively safe in the short-term, though there are no long-term studies regarding its use in adults with ID [5,30]. As for other hypnotics, if melatonin is unsuccessful there is usually little if anything to be gained from endlessly increasing the dose, and switching to a different class of hypnotics should be considered [31].
Vignette 1
A 36-year-old man with severe intellectual disability (non-verbal) and adult Attention Deficit Hyperactivity Disorder was referred by his intellectual disabilities psychiatrist for the investigation and management of treatment-resistant insomnia. He had additional co-morbidities of anxiety and type II diabetes, and was prescribed: metformin (500mg three times daily), propranolol (40mg twice daily; indication: anxiety) and methylphenidate extended release (60 mg once daily). The psychiatrist had trialled reducing methylphenidate and subsequently introduced modified release melatonin without success. Additional trials of promethazine (to 50mg) and mirtazapine (to 15mg) resulted in sleep deterioration. The patient typically retired to bed at 10pm, and carers described a markedly delayed sleep onset latency, during which time, he repeatedly left his bed and paced. On closer questioning, they described an inability to sit still, beginning each evening (around 8PM), even when they played his favourite television programme or music. Once asleep, there were no obvious difficulties with sleep maintenance, and his rising time was fixed for 9AM to coincide with structured daytime activities. During the day he was noted to be irritable, with worsening hyperactivity (which had not responded to higher stimulant doses). He had no history of anti-social snoring nor seizures, and he would not tolerate home nor inpatient sleep investigations. Based on the history, a clinical diagnosis of Restless Legs Syndrome (RLS) was made. RLS is the commonest mimic of insomnia, and in adults with ID, can present very similarly to this patient [32]. Moreover, medications with anti-histamine function, such as promethazine and mirtazapine can exacerbate RLS (and hence why his sleep deteriorated when these were trialled) [33]. It is also commonly co-morbid in adults with ADHD (~44%) [34] and type II diabetes [35]. The GP arranged for baseline blood tests, including a ferritin level and HbA1c. The ferritin level was <100mcg/L (the recommended lower limit for patients with RLS), which we addressed with Ferinject, when oral iron supplementation resulted in constipation. We withdrew his propranolol (beta-blockers can worsen RLS) [33], and as he remained symptomatic, slowly commenced Pregabalin, up-titrating to 150mg once nightly. He responded well to this, with an abolition of his evening restlessness, a normalised sleep onset latency, and there were notable improvements to his next day mood and function.
Vignette 2
A 21-year-old woman with Cornelia de Lange Syndrome (CdLS), and severe intellectual disability (non-verbal) was referred for the evaluation of sleep-disrupting nocturnal coughing, following an unremarkable respiratory medicine assessment. She had co-morbidities of constipation, for which she was prescribed lactulose, and had a past history of recurrent aspiration pneumonia, requiring a recent inpatient admission. Her parents reported intermittent coughing throughout the night, which at times was associated with emesis, and it disturbed her sleep maintenance. Daytime hyperactivity was notably worse following nocturnal coughing. Her GP had trialled a proton pump inhibitor (PPI) without success. Clinical assessment was not suggestive of a co-morbid sleep disorder, and home pulse oximetry was unremarkable. Despite the failure of a PPI, given her presentation and background of CdLS, we had a high index of clinical suspicion for gastro-oesophageal reflux disease (GORD). Stool analysis confirmed Heliobacter pylori (H. pylori), which when treated, resolved the nocturnal cough, improved sleep maintenance, and daytime hyperactivity reduced. 66% of individuals with CdLS experience GORD, and there is a strong correlation between the degree of oesophageal damage and the behavioural phenotype, with hyperactivity being the most common (up to 85%) [36, 37]. Moreover, worsening of behavioural symptoms is often used as a major sign of oesophageal damage in CdLS [38]. Nocturnal cough is a common sleep disrupter, which may cause neuropsychiatric disorders, and it negatively impacts on health-related quality of life (for review, see [39]).
Conclusion
Sleep disorders are common in adults with ID, and are important determinants of physical and mental health, as well as daytime function. Despite this, they are frequently overlooked, and not considered as primary diagnoses. There are many challenges when assessing, diagnosing, and treating sleep disorders in adults with ID, particularly when there is an overreliance on informant rather than subjective information. This is complicated by a lack of robust evidence regarding optimal adult ID sleep disorder management, as well as an often-significant reliance on care-giver/system ability and willingness to implement management strategies.
However, as our vignettes highlight, the benefits of successfully addressing sleep disorders in adults with ID far outweigh these challenges. Further research on sleep disorders in adults with ID is clearly required, including validated tools to aid primary and secondary care clinician screening, assessment and management. Only then will sleep medicine be able to retire Robert Sternberg’s quote – “if a child is labelled as having a learning disability, it has very concrete consequences for the kinds of services, …that child will get” [40].
References
- Shanahan P, Ahmad S, Smith K, Palod S, Fife-Schaw C. The prevalence of sleep disorders in adults with learning disabilities: A systematic review. British Journal of Learning Disabilities. 2022:1-24. https://doi.org/10.1111/bld.12480
- Korb L, et al. Sleep: the neglected life factor in adults with intellectual disabilities. BJPsych Bull, 2021: 1-7.
- Esbensen AJ, Schwichtenberg AJ. Sleep in Neurodevelopmental Disorders. Int Rev Res Dev Disabil. 2016;51:153-191. https://doi.org/10.1016/bs.irrdd.2016.07.005
- van de Wouw E, Evenhuis HM, Echteld MA. Prevalence, associated factors and treatment of sleep problems in adults with intellectual disability: a systematic review. Res Dev Disabil. 2012;33(4):1310-32. https://doi.org/10.1016/j.ridd.2012.03.003
- Wilson S, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: An update. J Psychopharmacol. 2019;33(8):923-947. https://doi.org/10.1177/0269881119855343
- Carr EG, et al. Using mood ratings and mood induction in assessment and intervention for severe problem behavior. Am J Ment Retard. 2003;108(1):32-55. https://doi.org/10.1352/0895-8017 (2003)108<0032:UMRAMI>2.0.CO;2
- Symons FJ, Davis ML, Thompson T. Self-injurious behavior and sleep disturbance in adults with developmental disabilities. Res Dev Disabil. 2000;1(2):115-23. https://doi.org/10.1016/S0891-4222(00)00028-7
- Medic G, M, Wille M, Hemels ME. Short and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151-161. https://doi.org/10.2147/NSS.S134864
- Brylewski J, Wiggs L. Sleep problems and daytime challenging behaviour in a community-based sample of adults with intellectual disability. J Intellect Disabil Res. 1999;43(Pt 6):504-12. https://doi.org/10.1046/j.1365-2788.1999.00234.x
- McCurry SM, Song Y, Martin JL. Sleep in caregivers:what we know and what we need to learn. Curr Opin Psychiatry. 2015;28(6):497-503. https://doi.org/10.1097/YCO.0000000000000205
- Knapp M, et al. Intellectual disability, challenging behaviour and cost in care accommodation: what are the links? Health Soc Care Community. 2005;13(4):297-306. https://doi.org/10.1111/j.1365-2524.2005.00539.x
- Thornton J. People with learning disabilities have lower life expectancy and cancer screening rates. BMJ. 2019;364:l404. https://doi.org/10.1136/bmj.l404
- Sateia MJ. International Classification of Sleep Disorders-Third Edition. Chest. 2014;146(5):1387-1394. https://doi.org/10.1378/chest.14-0970
- Firth J, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360-380. https://doi.org/10.1002/wps.20773
- NHS. Available from: https://www.nhs.uk/conditions/learning-disabilities/annual-health-checks/.
- Carvalho AA, et al. STOP-Bang questionnaire should be used in all adults with Down Syndrome to screen for moderate to severe obstructive sleep apnea. PLoS One, 2020;15(5):e0232596. https://doi.org/10.1371/journal.pone.0232596
- Cornacchia M, et al. The Prevalence of OSA Among an Adult Population With Down Syndrome Referred to a Medical Clinic. Am J Intellect Dev Disabil. 2019;124(1):4-10. https://doi.org/10.1352/1944-7558-124.1.4
- Trois MS, et al. Obstructive sleep apnea in adults with Down syndrome. J Clin Sleep Med. 2009;5(4):317-23. https://doi.org/10.5664/jcsm.27541
- Boudrea E.A, et al. Review of disrupted sleep patterns in Smith-Magenis syndrome and normal melatonin secretion in a patient with an atypical interstitial 17p11.2 deletion. Am J Med Genet A. 2009;149A(7):1382-91. https://doi.org/10.1002/ajmg.a.32846
- Surtees ADR, et al. Sleep duration and sleep quality in people with and without intellectual disability: A meta-analysis. Sleep Med Rev. 2018;40:135-150. https://doi.org/10.1016/j.smrv.2017.11.003
- McPherson P, Kaushal M, Kothapalli V. The Treatment of Dually Diagnosed Individuals with Sleep Disturbances and Intellectual Disabilities. Handbook of Dual Diagnosis, ed. J.Matson. 2020 Springer. https://doi.org/10.1007/978-3-030-46835-4_36
- Hill EA, et al. Utility of the pictorial Epworth sleepiness scale in the adult down syndrome population. Sleep Med. 2020;66:165-167. https://doi.org/10.1016/j.sleep.2019.10.003
- Pivetta B, et al. Use and Performance of the STOP-Bang Questionnaire for Obstructive Sleep Apnea Screening Across Geographic Regions: A Systematic Review and Meta-Analysis. JAMA Netw Open. 2021;4(3):e211009. https://doi.org/10.1001/jamanetworkopen.2021.1009
- O’Sullivan RBS, Hamilton A, et al. Concordance of objective and subjective measures of sleep in children with neurodevelopmental conditions: A systematic review and meta-analysis. Sleep Medicine Reviews. 2023;71. https://doi.org/10.1016/j.smrv.2023.101814
- Rosen A, D.O.P., et all, A comparison of sleep restriction and sleep compression on objective measures of sleep: A sub-sample from a large randomised controlled trial. Journal of Sleep Research, 2023. 32(4): p. e13826. https://doi.org/10.1111/jsr.13826
- Li C, Boon M, Ishman SL, Suurna MV. Hypoglossal nerve stimulation in three adults with down syndrome and severe obstructive sleep apnea. The Laryngoscope. 2019;129:E402-E406. https://doi.org/10.1002/lary.27723
- Sultana R et al. The Case for Early Use of Glucagon-like Peptide-1 Receptor Agonists in Obstructive Sleep Apnea Patients with Comorbid Diabetes and Metabolic Syndrome. Life (Basel) 2022;12(8) https://doi.org/10.3390/life12081222
- NICE. Available from: https://cks.nice.org.uk/topics/insomnia/.
- Pigeon WR. Treatment of adult insomnia with cognitive-behavioral therapy. J Clin Psychol. 2010;66(11): 1148-60. https://doi.org/10.1002/jclp.20737
- Braam, W., et al., Exogenous melatonin for sleep problems in individuals with intellectual disability: a meta-analysis. Dev Med Child Neurol. 2009;51(5):340-9. https://doi.org/10.1111/j.1469-8749.2008.03244.x
- Wilson SAK, Baldwin D, Dijk DJ, Espie A, Espie C, Gringras P, Krystal A, Nutt D, Selsick H, Sharpley A. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: An update. J Psychopharmacol. 2019;33(8):923-947. https://doi.org/10.1177/0269881119855343
- RCPsych. Available from: https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/college-reports/college-report-cr230—attention-deficit-hyperactivity-disorder-(adhd)-in-adults-with-intellectual-disability.pdf.
- O’Regan, D, Anderson KN. Restless legs syndrome and periodic limb movements of sleep. Br J Hosp Med (Lond). 2020;81(1):1-8. https://doi.org/10.12968/hmed.2019.0319
- Cortese S, et al. Restless legs syndrome and attention-deficit/hyperactivity disorder: a review of the literature. Sleep. 2005;28(8):1007-13. https://doi.org/10.1093/sleep/28.8.1007
- Skomro RP, et al. Sleep complaints and restless legs syndrome in adult type 2 diabetics. Sleep Med. 2001;2(5): 417-22. https://doi.org/10.1016/S1389-9457(01)00110-1
- Luzzani S, et al. Gastroesophageal reflux and Cornelia de Lange syndrome:typical and atypical symptoms. Am J Med Genet A. 2003;119A(3):283-7. https://doi.org/10.1002/ajmg.a.20191
- Jean O’Hara, Nick Bouras JM. Intellectual disability and ill-health. In Intellectual Disability and Ill Health: A Review of the Evidence (pp. I-Ii). 2010.
- Hall SS, et al. Health and sleep problems in Cornelia de Lange Syndrome: a case control study. J Intellect Disabil Res. 2008;52(Pt 5):458-68. https://doi.org/10.1111/j.1365-2788.2008.01047.x
- Singh DP, Jamil RT, Mahajan K. Nocturnal Cough, in StatPearls. 2023: Treasure Island (FL).
- Quotes. Available from: https://quotefancy.com/quote/1428622/Robert-Sternberg-So-for-example-if-a-child-is-labeled-as-having-a-learning-disability-it


