Few clinical scenarios have as much potential to evoke ill-feeling and inter-disciplinary tension as the agitated patient. Though unfortunate this is understandable. With agitation comes disruption of normally efficient ward routines, fear of aggression and even actual injury. Staff who feel threatened and ‘out of their depth’ struggle to provide optimal patient care, a problem potentially magnified when misinformation is seen as fact (see Table 1) and the view this ‘should be someone else’s problem’ gains purchase. The reality is alternative placements are rarely available for medically unwell agitated patients. Consequently tempers fray, a misinterpretation of ‘zero tolerance’ policies is used to justify disengagement, and the care of often particularly sick patients suffers with potentially devastating consequences. Thankfully however with adequate preparation staff can increase their confidence in working with these patients, derive much satisfaction from providing good quality care, and greatly improve outcomes.

Emergency management of agitation
Agitation is instantly recognisable, but harder to define. It encompasses both subjective distress and motor restlessness (pacing, wringing hands etc). Though crises such as aggression or attempts to abscond are what typically precipitate senior staff being summoned, they rarely occur without warning. Generally they follow a prodromal period of increasing irritability, possibly with glaring, fidgeting or pacing.1 Management of aggression training emphasises recognising this escalating agitation and intervening to avert crises. This involves calmly and empathically engaging the patient, attempting to ascertain the causes of their increasing agitation and attenuating them before overt aggression supervenes. Crucial questions are whether the patient is delirious or has a major psychiatric illness. Clearly however, this assessment must be done in tandem with planning for violence, guarding personal safety and ensuring adequate staff to restrain the patient should this prove necessary. Oral medication should be offered, but intramuscular medication also prepared. This will normally be Haloperidol, though if medical contraindications or alcohol/sedative withdrawal is suspected Lorazepam is preferred. Doses are determined more by medical fitness rather than level of agitation. A starting dose of Haloperidol 0.5mg (or Lorazepam 0.5mg if antipsychotics contraindicated but emergency tranquilisation essential) would be indicated in a frail elderly woman, whereas a psychotic young man may receive both Haloperidol 5mg and Lorazepam 2mg.
Assessment
In medical inpatients delirium is the cause of agitation until proved otherwise. It is characterised by an abrupt onset, altered conscious level and fluctuating course. Impaired attention, with associated disorientation, is the key clinical finding. It can be identified through simple bedside tests such as serial 7 subtractions or, still sensitive but less influenced by education, listing the months of the year backwards.2 Additional disturbances in cognition (particularly memory, executive and visuospatial functions) are also often present, sleep is fragmented, and perceptual disturbances, especially illusions and visual hallucinations, can occur. Previously regarded as a transient disturbance from which recovery was the norm, delirium is now recognised as a medical emergency. It is associated with high mortality, and even optimally treated can have long term consequences for survivors. Twenty percent of delirious patients still exhibit symptoms 3-6 months after onset,3 and it is associated with an increase in incident dementia.4 Prospective studies demonstrate this association after controlling for potential confounders, indicating delirium does not just predict dementia but directly contributes to cognitive decline. Animal studies suggest mechanisms by which this could occur, demonstrating that in animals with neurodegenerative disease systemic inflammation can have a neurotoxic effect.5 Prevention should be a hospital priority, and early detection and intervention is crucial. There is no shortage of well validated screening tools (e.g. the 4AT, www.the4at.com) and they should be more widely used; the ‘time is brain’ maxim may also be very applicable to delirium. Moreover, though the agitated patient is being discussed here, remember these are the minority of delirious patients; hypoactive or mixed presentations are more common. Hypoactive delirious patients often go unnoticed though they probably represent more severe disturbance with a greater likelihood of mortality.6
While the most likely cause of agitation in a hospital is delirium, there are other possibilities. These, together with features which distinguish them, are detailed in Table 2.
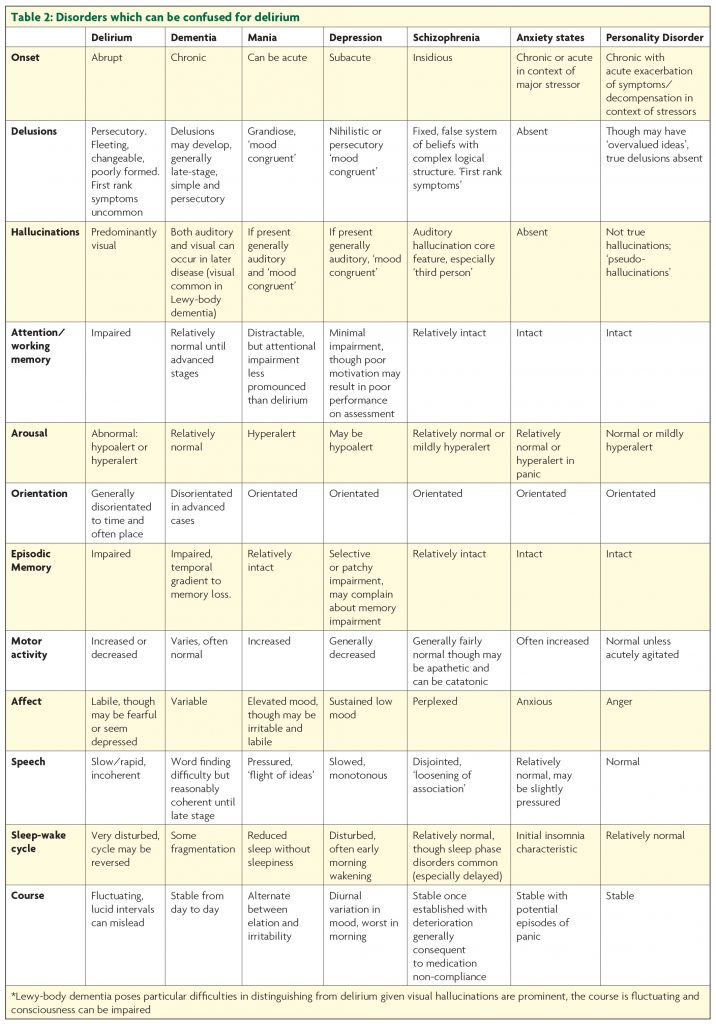
Agitation does of course occur in dementia, but with the exception of Lewy-body dementia hallucinations are generally not prominent until the advanced stages. Though they may not be orientated, demented patients are normally alert, onset is insidious rather than acute, and sleep cycle disturbance is much less pronounced than in delirium. Patients with schizophreniform or manic psychosis are generally orientated and have preserved recent memory. Though they may be distractible, they will not have the gross attentional disturbance of delirium. The lethargy and psychomotor retardation of hypoactive delirium can be confused for the avolition and withdrawal of severe depression.7 As with other ‘psychiatric’ conditions hallucinations in depression are usually auditory rather than visual. Like delusions they are mood congruent, and generally focused on ideas of guilt, death and decay.
On occasion agitation may result as an inappropriate response to a stressor in the absence of delirium or a major psychiatric illness. This may reflect the difficulties in interpersonal functioning and impulse control characteristic of personality disorders. If this is the diagnosis there will likely be an established history of similar conduct. Behaviour is regarded as volitional and may warrant removal from the ward and potentially prosecution. Keep in mind though that these patients can evoke very strong emotions which can cloud clinical judgement, and evidence of personality change should raise the possibility of an underlying neurological (or other medical) disorder. Withdrawal from a variety of abused substances can precipitate agitation. In the case of sedative agents (e.g. alcohol, benzodiazepines) this may precipitate delirium. Opiate withdrawal is associated with intense irritability, restlessness and craving; though treatment of withdrawal may be necessary to enable the patient to receive necessary medical treatment, it is not life threatening and consciousness is clear.
With a clear psychiatric diagnosis, transfer to psychiatric care is generally indicated. Behavioural disturbance in dementia (once delirium is excluded) is also generally managed in psychiatric settings. Though in exceptional (and relatively medically stable) cases risk may necessitate transfer to psychiatric care, delirious patients will overwhelmingly be managed on medical wards. It is important for physicians to appreciate just how limited the medical capabilities of psychiatric wards actually are. Mental health nursing is a distinct undergraduate degree from medical nursing, so it is unsurprising psychiatric nurses do not feel confident caring for medically unwell patients and intravenous administration of drugs and fluids is generally not feasible. Additionally resources for basic monitoring such as pulse oximetry are generally absent and even obtaining a chest X-ray often means travel to a different site.
Delirium
The treatment priority in delirium is identifying and addressing precipitants and maintaining factors. Multifactorial causation is the norm not exception, meaning consideration of potential contributors should not cease when a putative precipitant is identified. Remember that the threshold for developing delirium in compromised brains (be it because of dementia, MS, Parkinson’s disease, traumatic brain injury or even, possibly, depression8) is lowered. If very vulnerable, relevant precipitants (such as sleep disturbance, hunger or simply being in a strange environment) may seem trivial. Their potential importance can be understood however when delirium is conceptualised as arising through a complex interaction of pre-existing vulnerabilities, direct brain insults (such as drug effects and metabolic abnormalities) and aberrant stress responses. The latter are increasingly being elucidated and likely include aberrant HPA axis activity and primed microglia amplifying intracerebral inflammatory effects.9 Though heterogeneity in causation makes discussion of a ‘final common pathway’ in delirium facile, reduced acetylcholine and increased dopamine are often thought important.10
Treatment of delirium
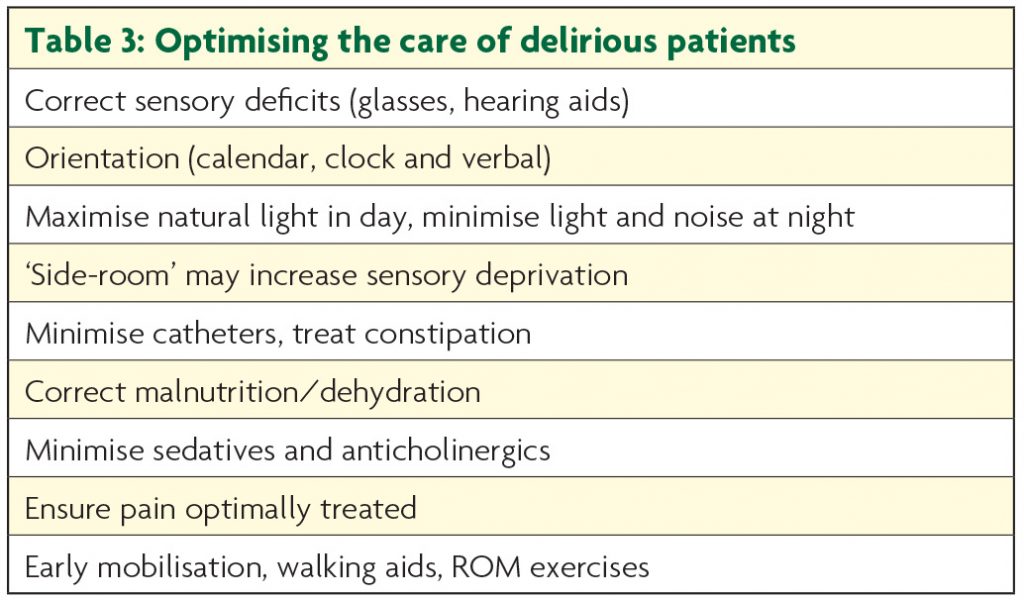
Optimising the environment is crucial. Many features of delirium-friendly care should be routine practice (see Table 3) and, though controlled studies are understandably hard to conduct, their implementation has been demonstrated to reduce delirium incidence.11
Distressed patients may require 1:1 nursing care, the ‘sitter’ providing orientation and reassurance with simple repeated statements e.g. ‘You are in hospital. You are safe.’ Family can be a useful resource, their effectiveness optimised by delirium education and guidance on how to interact with the patient.12
Medication can be helpful in delirium, but is not a substitute for optimising care. Antipsychotics are generally the agents of choice; NICE recommends Haloperidol or Olanzapine, though Risperidone is also a reasonable choice (Table 4).
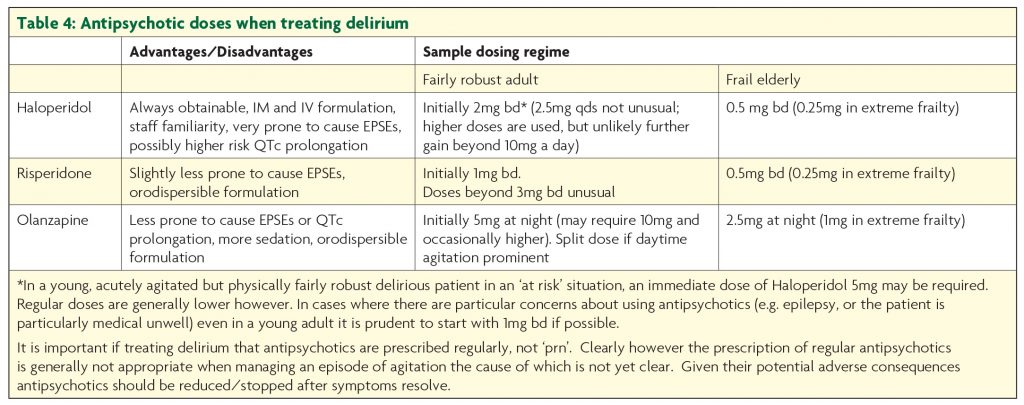
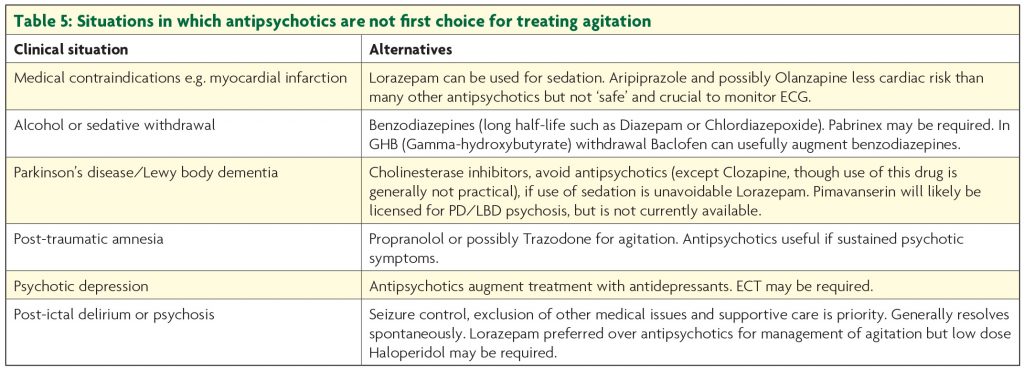
Though the latter two drugs may be associated with quicker response and are less likely to give rise to extrapyramidal side effects,13 in practice Haloperidol is generally first choice. Olanzapine is actually fairly anticholinergic which is a theoretical disadvantage; this does not generally cause problems, but its sedative properties mean it is not first choice in hypoactive delirium. All antipsychotics can cause akathisia, important to remember in patients becoming more restless as doses increase. The association between antipsychotic use in dementia and increased mortality is well established,14 so they should be stopped as soon as possible. Despite being the first drug identified to increase risk of stroke, the hazard ratio for mortality associated with Risperidone in demented patients may be less than with Haloperidol, and that with Olanzapine lower still.15 Though antipsychotics may augment treatment if psychotic symptoms are present, benzodiazepines are the cornerstone of treatment for delirium precipitated by withdrawal from sedatives such as alcohol or benzodiazepines. A typical regime for alcohol withdrawal would start with Diazepam 10mg four times a day, but doses are titrated to symptoms and may be much higher. Other circumstances when drugs other than antipsychotics are the first choice for agitation (due to delirium or other cause) are considered in Table 5.
Novel treatment options for delirium
Given the consistent finding of reduced acetylcholine in delirium, cholinesterase inhibitors would be expected to be helpful. Increased mortality when Rivastigmine was trialled as adjunctive treatment to Haloperidol in delirious ICU patients curbed initial enthusiasm.16 These extremely unwell patients with multiple organ failure are quite different from the typical delirious population however, and targeted use of anticholinergics may in time find a role. They may be most useful in delirious patients with a pre-existing cholinergic deficit, such as patients with Alzheimer’s disease. Given the prominence of sleep disturbance, Melatonin or even ‘bright light therapy’ may find a role.17,18 Dexmedetomidine, an alpha 2 agonist, is associated with reduced incidence of delirium when used for sedation in ICU; it decreases centrally mediated sympathetic activity and, if hypotensive/bradycardic side effects can be tolerated, may in time be used to treat delirium out-with ICUs.19
Conclusions
Agitation is a common problem throughout hospitals, with neurology patients being particularly vulnerable. Poor management can have disastrous consequences, but an engaged staff team adhering to some fairly common-sense principles can greatly improve outcomes. Prevention and early detection of delirium must be a priority. Though evidence for the use of antipsychotics in the treatment of delirium is good, skilled nursing care can make a huge difference and the centrality of this must be recognised and skill development supported.
References
1. Zeller SL, Rhoades RW. Systematic reviews of assessment measures and pharmacologic treatments for agitation. Clin Ther 2010;32:3:403-425.
2. O’Regan NA, Ryan DJ, Boland E, Connolly W, McGlade C, Leonard M, Clare J, Eustace JA, Meagher D, Timmons S. Attention! A good bedside test for delirium? J Neurol Neurosurg Psychiatry 2014;85:10:1122-1131.
3. Meagher D, Adamis D, Trzepacz P, Leonard M. Features of subsyndromal and persistent delirium. Br J Psychiatry 2012;200:1:37-44.
4. Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. The Lancet Neurology 2015;14:8:823-832.
5. Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JNP, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry 2009;65:4:304-312.
6. Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J Gerontol A Biol Sci Med Sci 2007;62:2:174-179.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med 1995;155:22:2459-2464.
8. Greene NH, Attix DK, Weldon BC, Smith PJ, McDonagh DL, Monk TG. Measures of executive function and depression identify patients at risk for postoperative delirium. Anesthesiology 2009;110:4:788-795.
9. Cunningham C, MacLullich AM. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun 2013;28:1-13.
10. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. The American Journal of Geriatric Psychiatry 2013;21:12:1190-1222.
11. Inouye SK, Bogardus Jr ST, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney Jr LM. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:9:669-676.
12. Martinez FT, Tobar C, Beddings CI, Vallejo G, Fuentes P. Preventing delirium in an acute hospital using a non-pharmacological intervention. Age Ageing 2012;41:5:629-634.
13. Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2015; doi:10.1136/jnnp-2015-311049
14. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:15:1934-1943.
15. Langballe EM, Engdahl B, Nordeng H, Ballard C, Aarsland D, Selbæk G. Short-and long-term mortality risk associated with the use of antipsychotics among 26,940 dementia outpatients: a population-based study. The American Journal of Geriatric Psychiatry 2014;22:4:321-331.
16. Van Eijk M, Roes K, Honing M, Kuiper MA, Karakus A, Van der JAgt M, Spronk PE, Van Gool WA, Van der Mast, Roos C, Kesecioglu J. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Critical Care 2011;15:1:1-190.
17. Al-Aama T, Brymer C, Gutmanis I, Woolmore-Goodwin SM, Esbaugh J, Dasgupta M. Melatonin decreases delirium in elderly patients: A randomized, placebo-controlled trial. Int J Geriatr Psychiatry 2011;26:7:687-694.
18. Yang J, Choi W, Ko Y, Joe S, Han C, Kim Y. Bright light therapy as an adjunctive treatment with risperidone in patients with delirium: a randomized, open, parallel group study. Gen Hosp Psychiatry 2012;34:5:546-551.
19. Maldonado JR, Wysong A, van der Starre, Pieter JA, Block T, Miller C, Reitz BA. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics 2009;50:3:206-217.
