Summary
- The entorhinal cortex and hippocampus are key components of the brain’s spatial memory network and are affected from the initial stages of Alzheimer’s disease (AD).
- Testing of spatial memory is a sensitive measure of early AD.
- Determination of disease effect on grid cell and place cell function, and of their behavioural correlates, will facilitate studies of disease mechanisms and development of future diagnostic tests.
Introduction
The hippocampal formation, comprising the entorhinal cortex and hippocampus proper (the dentate gyrus and Cornu Ammonis subfields), is the first brain region to exhibit neurodegeneration in Alzheimer’s disease (AD) and determination of AD-related alterations in hippocampal structure and function is central to AD diagnosis. In addition to its role in episodic memory there is extensive evidence of hippocampal involvement in spatial memory, dating back to the discovery of spatially-related firing activity of hippocampal “place cells” in freely moving animals. This work, alongside the more recent discovery of “grid cells” in the entorhinal cortex with periodic firing patterns during spatial exploration, led to the award of the 2014 Nobel Prize for Medicine or Physiology to Professors John O’Keefe of University College London, May-Britt and Edvard Moser of the Norwegian University of Science and Technology, Trondheim, Norway, “for their discovery of cells that constitute a positioning system within the brain”.
Here we describe in brief the role of the hippocampal formation in spatial memory and the implications for AD. Episodic memory, and parietal contributions to “getting lost”, will not be covered in this article.
The neural basis of spatial behaviour
Egocentric and allocentric spatial representations
The cortical processing of sensory information to generate representations of space is a prerequisite for spatial memory. Data are initially processed within single-modality primary and secondary cortices, and subsequently integrated within multimodal association cortices. The widely cited model of Ungerleider and Mishkin (1982)2 posits that further information processing occurs along anatomically divergent pathways; information regarding objects is conveyed via an occipitotemporal pathway (the ventral “what” pathway) whereas information regarding space is mediated by an occipitoparietal pathway (the dorsal “where” pathway). At this stage spatial representation is egocentric, i.e. head-centred, in nature. A further stage of processing is required, possibly occurring within the retrosplenial cortex, to transform the representation of space from egocentric to allocentric, i.e. not centred on the person (or animal), with an attendant shift from a polar coordinate to a Cartesian coordinate system. Both forms of spatial representation may be used in navigational strategies, depending on the complexity of the route and environment; for instance, a direct navigation from A to B along a line of sight may be mediated by an egocentric (“follow your nose”) approach whereas navigation within more complex environments may be more efficiently undertaken if those environments are mapped according to an allocentric framework.
Single cell studies
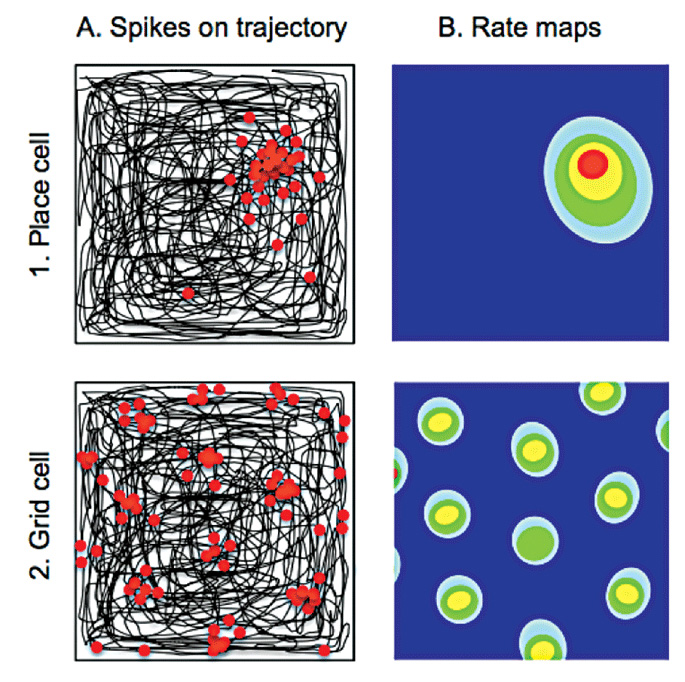
Column A shows in black the path taken by a rat as it traverses a square arena. Electrodes implanted within the hippocampus and entorhinal cortex record from individual neurons. Place and grid cells show increased firing (each action potential represented by a red dot) at discrete locations in the environment. Whereas individual place cells (top) fire only in one location, grid cells (bottom) have multiple firing fields. The hexagonal symmetry of the spacing between these latter fields gives rise to the term “grid cells”. The firing frequency of place and grid cells within the environment (rate maps) is shown in Column B, with lower wavelength colours (yellow and red) depicting higher rates of firing on a background of silent cell activity (dark blue).
Accurate representations of space, and spatial memory, are fundamental to the survival of most mammalian species. Professor O’Keefe’s discovery of “place cells” in the hippocampi of freely moving rodents during natural exploratory behaviour provided the first evidence of allocentric spatial representations within the mammalian brain.1 “Place cells” fire in a particular location in any given environment thus encoding an animal’s location;3 the location in which a place cell fires is known as its place field (Figure 1).
Later experiments demonstrated further characteristics of place cell firing including a robust correlation between place cell activity and spatial memory4 and the firing of individual place cells in different locations within different environments (place cell remapping).5 Place cell firing is observed in preweanling rats prior to any significant exploration of the environment6 supporting the Kantian notion of an innate spatial framework.7 Finally, in a transgenic mouse model of AD (Tg2576), disruption of place cell firing was found to correlate with impairment of spatial memory and with amyloid burden.8
Following the discovery of place cells, O’Keefe and Nadel proposed that the hippocampal formation also contained information regarding direction and distance allowing construction of a cognitive map of the environment.9 This postulated the existence of other spatially-tuned cells and, in line with this and other theoretical predictions, “grid cells” were identified in the entorhinal cortex in 2005 by May-Britt and Edvard Moser. Unlike place cells, which fire in a single location in any given environment, “grid cells” have periodic firing fields arranged with sixfold symmetry (Figure 1).10 Other cells with spatially-related firing activity have also been identified; “head direction cells” fire when the animal is facing in a particular direction whereas “boundary vector cells” fire in the proximity of a boundary, such as the edge of an environment. (For a comprehensive review of these different cell types and their respective functions, see reference 5).
Spatially-related single cell activity has been found in the hippocampal formation of humans and other mammals. Depth electrode recordings from epilepsy patients prior to temporal lobe surgery have revealed place cell and grid cell-like activity in the hippocampus and entorhinal cortex respectively11 and three-dimensional place and grid cell activity have been recorded in flying bats.12
Brain regions
Different subdivisions within the hippocampal formation underpin separate components of spatial behaviour. Within the hippocampus proper animal and human studies support the notion of functional differentiation along an anteroposterior axis, with anterior regions encoding contextual information and spatial novelty and posterior regions implicated in the storage of spatial representations. The entorhinal cortex is the primary source of afferents to the hippocampus, with medial and lateral subdivisions conveying spatial and object-related information respectively.13 The differing information content of these entorhinal inputs reflect the afferents to the medial and lateral entorhinal cortex from the parahippocampal cortex and perirhinal cortex, involved respectively in scene and object recognition.
A number of other brain regions subserve spatial processing. Mention has been made in passing of the role of parietal lobe regions, such as the retrosplenial cortex, which may additionally encode landmark information,14 and the precuneus and posterior cingulate gyrus are of particular interest given the early manifestation of AD pathology in these regions. Finally there is evidence for a striatal system for landmark-related representations of space.15
Tests of spatial memory
Perhaps the best known test of allocentric spatial memory is the Morris water maze,16 used extensively in preclinical phases of AD treatment trials. In this paradigm rodents have to remember the location of a hidden underwater platform within a pool of opacified (milky) water, on the basis of external sensory cues around the maze periphery. Other tests of spatial memory in animal models include continuous Y-maze alternation, forced-choice T-maze alternation, the radial arm water maze and the circular platform maze.
Test of allocentric spatial memory in humans include the 4 Mountains Test (Figure 2), which uses computer-generated mountain landscapes and is sensitive to focal hippocampal damage,17 and the Hidden Goal Task, which assesses memory for hidden locations within a three metre circular velvet arena.18 Other tasks include The Heading Orientation Test, The Money Road Map Test and virtual reality tasks including a radial maze task.19

Impairment of spatial memory in early AD
Several studies have demonstrated impairment of spatial memory in AD, as assessed by route learning tasks and memory for scenes.20-22 Both allocentric and egocentric spatial memory are impaired in AD18,20 with one study finding that AD subjects were more impaired when using an allocentric, as opposed to an egocentric, wayfinding strategy.23 Patients with AD also appear less able to translate between allocentric and egocentric representations, possibly reflecting damage to the retrosplenial cortex.24 Performance on the 4 Mountains Test differentiates patients with AD from those with non-AD dementias22,25 and more recently has been found to discriminate MCI patients with and without CSF biomarker evidence of underlying AD.26 Other studies have also demonstrated impairment of allocentric and egocentric memory in Mild Cognitive Impairment (MCI).18,21,27
Structure-function studies have revealed an association between hippocampal volumes and spatial memory performance, with a positive correlation noted in MCI and AD patients.26 In another MCI/AD patient study poor navigational performance and poor accuracy locating landmarks were associated respectively with a reduction in right hippocampal and posterior parietal volumes.21
Conclusions and future direction
The 2014 Nobel Prize for Medicine or Physiology was awarded in recognition of the work undertaken in elucidating the role of the hippocampus and entorhinal cortex in the representation of space. Knowledge of the spatially-related firing properties of place cells and grid cells, and of the coupled behaviours in the form of spatial exploration and memory, provide new opportunities for studying the initial effects of AD on brain function. Such study will not only provide a platform for systems biology investigations of disease mechanisms but will also aid development of new behavioural tasks with increased sensitivity for the earliest stages of AD.
References
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171-5.
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle, Goodale & Mansfield, editor. Analysis of Visual Behavior. Cambridge: The MIT Press; 1982. p. 549-86.
- O’Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381(6581):425-8.
- O’Keefe J, Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res. 1987;68(1):1-27.
- Hartley T, Lever C, Burgess N, O’Keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc Lond B Biol Sci. 2014;369(1635):20120510.
- Wills TJ, Cacucci F, Burgess N, O’Keefe J. Development of the hippocampal cognitive map in preweanling rats. Science. 2010;328(5985):1573-6.
- Kant I. The Critique of Pure Reason; 1781.
- Cacucci F, Yi M, Wills TJ, Chapman P, O’Keefe J. Place cell firing correlates with memory deficits and amyloid plaque burden in Tg2576 Alzheimer mouse model. Proc Natl Acad Sci U S A. 2008;105(22):7863-8.
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; 1978.
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436(7052):801-6.
- Jacobs J, Weidemann CT, Miller JF, Solway A, Burke JF, Wei XX, et al. Direct recordings of grid-like neuronal activity in human spatial navigation. Nat Neurosci. 2013;16(9):1188-90.
- Yartsev MM, Ulanovsky N. Representation of three-dimensional space in the hippocampus of flying bats. Science. 2013;340(6130):367-72.
- Reagh ZM, Yassa MA. Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc Natl Acad Sci U S A. 2014;111(40):E4264-73.
- Auger SD, Maguire EA. Assessing the mechanism of response in the retrosplenial cortex of good and poor navigators. Cortex. 2013;49(10):2904-13.
- Doeller CF, King JA, Burgess N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc Natl Acad Sci U S A. 2008;105(15):5915-20.
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47-60.
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, et al. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17(1):34-48.
- Laczo J, Vlcek K, Vyhnalek M, Vajnerova O, Ort M, Holmerova I, et al. Spatial navigation testing discriminates two types of amnestic mild cognitive impairment. Behav Brain Res. 2009;202(2):252-9.
- Bohbot VD, Lerch J, Thorndycraft B, Iaria G, Zijdenbos AP. Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. J Neurosci. 2007;27(38):10078-83.
- Cherrier MM, Mendez M, Perryman K. Route learning performance in Alzheimer disease patients. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(3):159-68.
- DeIpolyi AR, Rankin KP, Mucke L, Miller BL, Gorno-Tempini ML. Spatial cognition and the human navigation network in AD and MCI. Neurology. 2007;69(10):986-97.
- Bird CM, Chan D, Hartley T, Pijnenburg YA, Rossor MN, Burgess N. Topographical short-term memory differentiates Alzheimer’s disease from frontotemporal lobar degeneration. Hippocampus. 2010;20(10):1154-69.
- Kalova E, Vlcek K, Jarolimova E, Bures J. Allothetic orientation and sequential ordering of places is impaired in early stages of Alzheimer’s disease: corresponding results in real space tests and computer tests. Behav Brain Res. 2005;159(2):175-86.
- Morganti F, Stefanini S, Riva G. From allo- to egocentric spatial ability in early Alzheimer’s disease: a study with virtual reality spatial tasks. Cogn Neurosci. 2013;4(3-4):171-80.
- Pengas G, Patterson K, Arnold RJ, Bird CM, Burgess N, Nestor PJ. Lost and found: bespoke memory testing for Alzheimer’s disease and semantic dementia. J Alzheimers Dis. 2010;21(4):1347-65.
- Moodley KK, Minati L, Contarino VE, Prioni S, Wood R, Cooper R, et al. Diagnostic differentiation of mild cognitive impairment due to Alzheimer’s disease using a hippocampus-dependent test of spatial memory. Hippocampus. 2015 Jan 20. doi: 10.1002/hipo.22417. [Epub ahead of print]
- Weniger G, Ruhleder M, Lange C, Wolf S, Irle E. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia. 2011;49(3):518-27.

