Abstract
Relatively few patients with head injuries require the operative skills of a neurosurgeon. However, concerns that some patients with head injury die unnecessarily and other patients suffer long-term sequelae due to inappropriate management were raised in a report from the Royal College of Surgeons of England in 1999 [1]. This highlighted inadequacies in the provision of head injury services for these patients, many of whom are initially, and sometimes exclusively seen by relatively junior doctors. The National Institute of Clinical Excellence has published admission, CT scan and specialist referral guidelines [2]. Neurosurgeons should take a leading role by ensuring access to specialist care is appropriate and timely in order to be effective, and by closely collaborating with other health care workers to ensure an optimal pathway of care is organised.
Epidemiology
Head injuries occur in all age groups, with a peak incidence in males between the ages of 16 and 25 years and a second peak in the elderly who have a high incidence of chronic subdural haematomas. The majority of head injuries in the UK are closed rather than open and are caused by road traffic accidents, falls and assaults [3].
Haematoma evacuation
The Glasgow Coma Scale is a validated measure of level of consciousness that has been universally adopted [4]. It is of paramount importance in communicating information about patient status. The age, neurological status, mechanism of injury and pupillary reactions all input to the decision-making process when evaluating the need for haematoma evacuation. The major questions addressed by the surgeon are:
1) Is the conscious level depressed?
2) Are there other signs of raised ICP (eg oculomotor palsy)?
3) Does the scan show a haematoma or contusion with mass effect (midline shift/ ventricle effacement/ dilatation of contralateral ventricle/ loss of basal cisterns/ sulcal effacement)?
4) Is the expansion of the mass anticipated (eg contusions)?
5) Where is the mass (temporal lobe lesions are considered dangerous due to the association with uncal herniation compressing the midbrain)?
These questions must be rapidly addressed and any surgery performed with an appropriate sense of urgency. The majority of procedures require a large craniotomy flap. This provides adequate access to traumatised brain, potential sources of haemorrhage and can prove useful in reducing ICP if the bone flap is left out. The classical scalp incision for many lesions extends in a question mark shape to the pre-auricular region. Modification such that the posterior limb of the flap passes posterior to the ear increases the ease of exposing the temporal and parietal regions. Extradural haematomas are usually readily evacuated and bleeding is controlled by placement of multiple “hitch” stitches around the periphery of the bone flap and a central hitch stitch from the dura through 2 drill holes in the bone flap. Acute subdural haematomas after low impact injury in the elderly are often associated with a readily coagulated small cortical artery. After a high-velocity injury, an acute subdural haematoma is frequently associated with contusions and reflects a severe brain injury. In extreme cases, a “burst” lobe may be evident. Such severe haemorrhagic contusions may necessitate resection of the brain in the form of a lobectomy. Brain resection requires rapid but careful surgery preserving the middle and anterior cerebral arteries. Haemostasis can be difficult to achieve during trauma surgery. Good illumination, excellent access and patience are required. If the underlying brain is swollen at the time of closure it is prudent to leave the dura open, not replace the bone flap and close the scalp in anatomical layers. A subdural multilumen capillary drain (Yates) tunnelled to a colostomy bag applied to the scalp provides a very effective means of wound drainage. An ICP monitor is inserted in all cases with the exception of uncomplicated extradural haematoma evacuation where rapid recovery is anticipated.
Chronic subdural haematomas usually present with headache, confusion, focal neurological signs or reduced level of consciousness several weeks after minor head trauma. Frequently there is no history of trauma. The haematoma is drained through 2 burr-holes that also permit intraoperative irrigation of the subdural space to remove any clot debris.
Critical care management of head injury
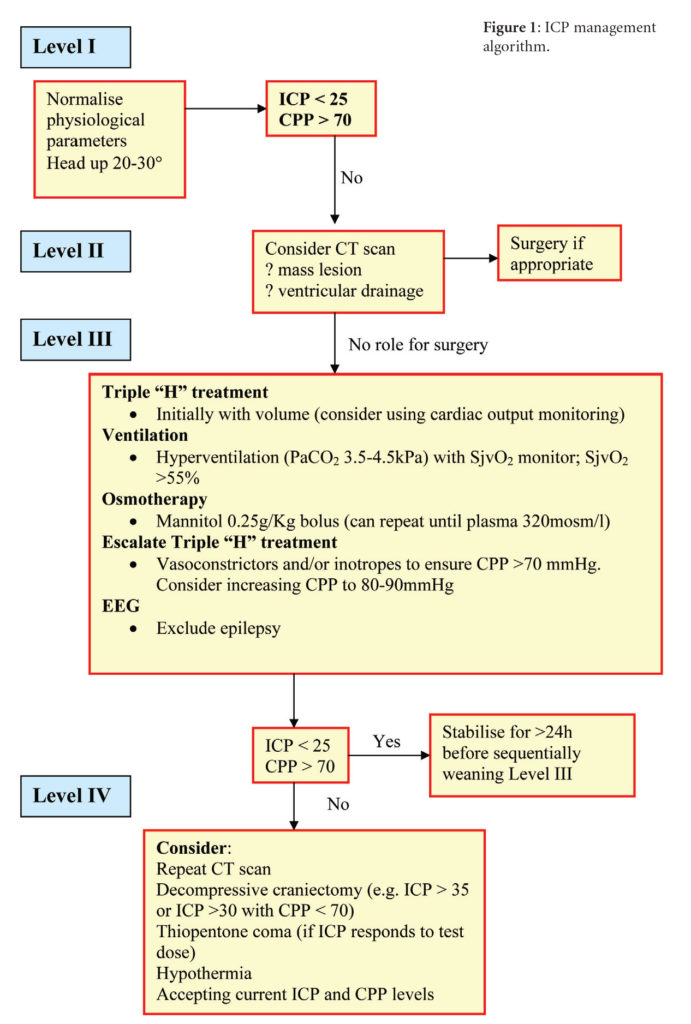
After the initial assessment and resuscitation of patients with craniocerebral trauma – including the management of life-threatening injuries – a period of intensive supportive care with multimodality monitoring is instituted to prevent secondary insults to the brain. Many centres now use protocol-driven therapy during this phase of care (Figure 1).
Monitoring
Intracranial pressure monitoring is used in all head-injured patients with any abnormality on CT scanning who do not obey commands. The monitor is usually placed in the right frontal region via a twist drill hole. Since an external ventricular drain is placed in over 50% of severely injured patients the exact positioning of the ICP monitor needs to be able to accommodate a nearby ventriculostomy incision.
Intracranial pressure and cerebral perfusion pressure management
Continuous monitoring of arterial blood pressure and calculation of mean cerebral perfusion pressure (CPP) is performed (CPP = mean arterial BP – mean ICP) [5]. Management of the ICP is undertaken in accordance with the algorithm in Figure 1. An ICP of <25 mmHg and a mean CPP of >70 mmHg are targeted. Simple manoeuvres frequently used to reduce intracranial pressure include ensuring venous drainage is not obstructed by endotracheal tube tapes, the elevation of the head by 20-30o, avoidance of hypoxia and prolonged expiratory cycles and maintaining normothermia. If the ICP is raised CT scans will exclude significant haematomas or contusions. Ventriculomegaly at this early stage is unusual but the placement of an EVD will usually reduce ICP if the ventricles are not fully effaced. If the ventricular catheter has not resulted in CSF at a depth of 7cm the trajectory is incorrect. Check that the entry point is not too far anterior (should be just anterior to the coronal suture), ensure that in the AP plane the tip is directed to the tragus and direct the catheter from the entry site toward the midline at the target depth, in the coronal plane. The catheter is tunnelled posteriorly to keep it clear of any subsequent bifrontal craniectomy flap.
PaCO2 manipulation and jugular venous oximetry (sJvO2)
The next phase of care involves further monitoring of the patient with jugular venous oximetry and the use of inotropic support [6]. Reduction of the arterial PaCO2 can reduce the intracranial blood volume due to capacitance vessel constriction reducing intracranial pressure. However, preliminary cerebral blood flow PET data indicate that such an approach can cause arterial vasoconstriction and relative cerebral ischaemia. Jugular venous oximetry is therefore used as a tool to titrate hyperventilation to the global cerebral metabolic response. If sJvO2 reduced below 60% a state of relative ischaemia exists and this is likely to be exacerbated by further reduction of PaCO2.
Inotropic support
Augmentation of the mean arterial pressure may help the delivery of oxygen and glucose to the brain averting the progression of cytotoxic oedema and reducing ICP. However intracranial pressure may increase with pressure support due to the loss of cerebral autoregulation, resulting in a passive cerebral circulation in which increases in arterial pressure causes further elevation of ICP due to pressure waves and vasogenic oedema. In clinical practice the response is variable, therefore therapeutic manoeuvres require careful monitoring. Augmentation of the CVP to 8 or 10cm ensures adequate circulatory volume is present before enhancing stroke volume with inotropes. Norepinephrine appears to be more predictable and efficient at augmenting CPP when compared with dopamine [7].
Osmotherapy
The reduction in ICP following mannitol administration has been well documented [8]. The mode of action has traditionally been ascribed to the osmotic diuretic effect by which water follows the osmotic gradient from the injured brain to vascular compartment across the bood-brain-barrrier. Other postulated mechanisms of action include a free radical scavenging effect, volumetric augmentation and improved capillary flow due to the rheological properties of mannitol. Other osmotic agents such as hypertonic (7.5%) saline are also undergoing clinical trials to establish whether they have a validated therapeutic role in the management of raised ICP.
Hypothermia
Hyperthermia increases the metabolic rate of brain tissue and is associated with a poorer outcome from head injury. However, a recent large multicentre randomised trial of hypothermia in the management of a heterogenous head injured population has not established a case for the widespread use of this therapy [9]. The selective use of hypothermia may be appropriate in some subgroups of patients, for example, those with raised ICP.
Refractory elevation of ICP
Refractory elevation of ICP can produce a stalemate situation during the intensive care management of head-injured patients. Secondary insults such as hypoxia and hypovolaemia can lead to an acutely raised ICP and reduction in CPP that is life-threatening. A therapeutic decompressive craniectomy increasing the volumetric capacity of the cranial cavity can significantly reduce the ICP and may be beneficial in this group of patients [10]. The RESCUE trial is evaluating this procedure.
Barbiturates reduce cerebral metabolic demand and it has been postulated reduce cytotoxic oedema. However, their use is controversial with a poorly defined evidence base [11]. Thiopentone is cardiotoxic and induces a prolonged comatose state that precludes clinical assessment of the conscious level and makes treatment withdrawal a protracted issue. To avoid inappropriate use, an infusion is only considered if ICP elevation is refractory and a reduction in ICP is observed after administering a test dose.
Outcome after head injury
Neuropsychological deficits
Survival with a moderate or severe disability is common after all grades of head injury. Even after mild injury 47% of patients have disabling persistent neuropsychological deficits [12]. Frontal lobe dysfunction occurs most commonly resulting in loss of motivation and drive, increased fatiguability, concentration and attention deficits, defective problem solving and impaired cognitive endurance. Coupled with a lack of insight and loss of social judgement severe social handicaps can result. Temporal lobe damage causes memory deficits, word-finding difficulties, irritability, mood lability, depression, anxiety and a loss of self-esteem. SSRIs may be useful in managing these complications. Unfortunately, only a minority of these patients receive help from a specialist in rehabilitation, a clinical neuropsychologist or social services. Improved provision of rehabilitation facilities is needed to minimise the cognitive and social sequelae of injury. Assessment of rehabilitation needs is required at an early stage by all patients with intermediate and severe head injuries and transfer to a well-resourced rehabilitation centre with physiotherapy, speech and language therapy, occupational therapy and neuropsychology is appropriate when patients are medically and surgically stable. Although some severely disabled or vegetative patients will ultimately require transfer to a nursing care facility such a decision should not be made precipitously.
Post-traumatic epilepsy
Ventilated head-injured patients can sustain occult seizure activity that is a potential cause of raised intracranial pressure. EEG monitoring to exclude fits as a contributory factor is therefore appropriate in patients with refractory raised ICP.
Overall, up to 5% of patients sustain early post-traumatic epilepsy – defined as seizures within 1 week of the injury. Usually, such patients are administered intravenous phenytoin at, or soon after, the time of the fit. The majority of these patients do not develop long-term epilepsy and prolonged use of anticonvulsants is not beneficial [13].
The incidence of long-term post-traumatic epilepsy is influenced by the type and severity of the brain injury. Depressed fractures with dural tears and intraparenchymal injuries are both associated with long-term epilepsy in over 25% of cases. 50% of these patients will have their first fit within 1 year of the injury with over 80% having fits within 4 years of the injury. However, a significant minority will sustain the first seizure more than a decade after the trauma [14]. Established anticonvulsants such as sodium valproate or carbamazepine are appropriate first-line drugs in these patients.
Care pathways
Standardisation of the care system for head-injured patients requires a multi-faceted approach with an increase in resources. Renovation and expansion of accident and emergency departments with the provision of observation beds has been recognised as a nationwide requirement and is underway. An improvement in the infrastructure of radiological departments, with fully staffed CT scanners and image transfer facilities, is required. More neurosurgical intensive care beds and rehabilitation facilities are required in almost all regions. The health and social service departments must work in partnership to serve the needs of patients and their families. Continuing medical education programmes for doctors in all specialities that are involved in head injury care need to include sessions allocated to head injury management, to ensure improvements in management are incorporated into clinical practice.
References
- Royal College of Surgeons of England. Report of the working party on the management of patients with head injuries. http://www.rcseng.ac.uk/services/publications/publications/pdf/rep_hi.pdf 1999;
- NICE. Head injury – triage, assessment, investigation and early management of head injury in infants, children and adults. http://www.nice.org.uk/pdf/headinjury_full_version_completed.pdf 2003;
- Jennett B. Epidemiology of head injury. J Neurol Neurosurg Psychiatry 1996;60:362-9. https://doi.org/10.1136/jnnp.60.4.362
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;ii:81-4. https://doi.org/10.1016/S0140-6736(74)91639-0
- Rosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg 1995;83:949-62. https://doi.org/10.3171/jns.1995.83.6.0949
- Cruz J. The first decade of continuous monitoring of jugular bulb oxyhemoglobin saturation: management strategies and clinical outcome. Critical Care Medicine 1998;26:344-51. https://doi.org/10.1097/00003246-199802000-00039
- Steiner LA, Johnston AJ, Czosnyka M, et al. Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head-injured patients. Critical Care Medicine 2004;32:1049-54. https://doi.org/10.1097/01.CCM.0000120054.32845.A6
- Mendelow AD, Teasdale GM, Russell T, Flood J, Patterson J, Murray GM. Effect of mannitol on cerebral blood flow and cerebral perfusion pressure in human head injury. J Neurosurg 1985;63:43-8. https://doi.org/10.3171/jns.1985.63.1.0043
- Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. New Engl J Med 2001;344:556-63. https://doi.org/10.1056/NEJM200102223440803
- Whitfield PC, Patel H, Hutchinson PJ, et al. Bifrontal decompressive craniectomy in the management of posttraumatic intracranial hypertension. Br J Neurosurg 2001;15:500-507. https://doi.org/10.1080/02688690120105110
- Ward JD, Becker DP, Miller JD, et al. Failure of prophylactic barbiturate coma in the treatment of severe head injury. J Neurosurg 1985;62:383-8. https://doi.org/10.3171/jns.1985.62.3.0383
- Thornhill S, Teasdale GM, Murray GM, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: prospective cohort study. Br Med J 2000; 320:1631-5. https://doi.org/10.1136/bmj.320.7250.1631
- Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomised double blind study of phenytoin for the prevention of post-traumatic seizures. New Engl J Med 1990;323:497-502. https://doi.org/10.1056/NEJM199008233230801
- Jennett B. Early traumatic epilepsy. Incidence and significance after nonmissile injuries. Arch Neurol 1974;30:394-8. https://doi.org/10.1001/archneur.1974.00490350052008
